Document Type : Original Research Article
Authors
1 Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran
2 Department of Chemistry, Quchan Branch, Islamic Azad University, Quchan, Iran
Abstract
In this work, we introduced the fabrication of a novel modified Carbon Paste Electrode (CPE) for the determination of Lanthanum ion based on Multi-Walled carbon nanotubes. The CPE combination contained paraffin, graphite and N, N-Bus (salicyliden)-1,3-propandiamine (SB-SalPr) that we reemployed in the structure of electrode. After specifying the optimal percentages, this electrode showed the Nernstian response with a slope of 19.37 (mV/decade) to La3+ ion in response time (
Graphical Abstract
Keywords
Main Subjects
Introduction
Ion selective electrodes are chemical sensors that respond selectively to active ions [1-2]. Lanthanum has a low to moderate level of toxicity; therefore, we should be careful. By changing and miniaturize the potentiometric ion electrode, a paste electrode was made that no longer needed an internal solution. These electrodes have the appropriate texture to accommodate modifying compounds from renewable surfaces. Carbon paste electrodes are widely used in electrochemical studies due to their lower detection limit and higher ohmic resistance, easy surface renewal and miniaturization convenience [3-6].
Since the discovery of carbon nanotubes by Enigma, much research has been done on the structure of carbon nanotubes and their physical and chemical characteristics. Carbon nanotubes have mechanical strength, high flexibility, chemical inertness, conductivity, and the ability to act on the external surface. Research shows that CNTs apply a good electro-catalyst effect and their electron transfer rate is high [7-11].
Therefore, carbon nanotubes are widely used in chemical and biochemical sensors today [12-16]. Also, carbon nanotubes are commonly used in sensors to modify the linear range and reduce the response time of electrodes [17,18].
Nano-tubes are divided into two main groups attainable today. Single-Walled Carbon nano-Tubes (SWCNTs) have a single sheet of grapheme; it rolls uniformly to form o cylinder with diameter of order of 1 nm of length of up to centimeters [19,20].
Multi-Walled Carbon Nan-Tubes (MWCNTs) have an array of such cylinder organized concentrically and separated by 0.35 nm; it is similar the basal plane separation in graphite [21].
The diameter of MWCNTs ranges from 2 to 100 nm and their length range is tens of microns. In this work, a new nanocomposite-based modified CPE was built for La3+cation determination and potentiometric titration versus EDTA.
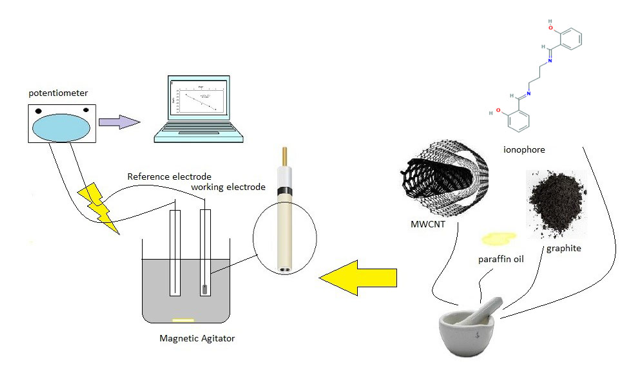
Generally charged ionophores or neutral ligands are commonly applied to make electrodes sensitive to ions. The ligand structure in this work was N, N-Bis (salicyliden)-1,3-propandiamine (SB-SalPr). (Figure 1).
Experimental
Chemicals and reagents
Graphite powder with a <50 μm particle size (Merck) and paraffin oil (Aldrich) was used for Multi-Walled Carbon Nano-Tubes (MWCNTs) whit 20-30 nm diameters, 10-30 μm length was bought from Iranian Nanomaterial’s pioneers company. The nitrate salts of the cations and N, N-Bis (salicyliden)-1,3-propandiamine (SB-SalPr) were purchased from Merck Chemical. Deionized water was used for throughout.
Instruments
The reference electrode was the electrochemical cell included calomel (Azar Electrode); the indicator electrode was the Lanthanum (III) sensor. The structure of sensor is as follows:
Carbon Paste Electrode | sample solution |Hg- Hg2Cl2, KCl (sat’d).
A corning ion analyzer 250 pH/mV meters was applied for the potential measurements at 25.0 ℃. The logarithm of the concentration of the lanthanum ion was used to determine and plot the potential.
Carbon Paste electrode preparation
After mixing the intended dosage of SB-SalPr, graphite powder, paraffin and functionalized Nano-Tubes completely, the mixture was transferred into a glass tube with i.d. 5mm and height of 3 cm. To prevent air gaps to form, the paste was packed into the tube following the homogenization of the mixture. This step was taken to impede the heightened the electrical resistance of the electrode. A copper wire was appended into the opposite end. A soft abrasive paper was used to polish the working surface of the electrode; then, to condition the electrode, it was presoaked in a 1.0×10-3 mol L-1 of La (NO3)3 .6H2O solution for 24 h [22-26].
Results and discussion
Potential response of the electrode
In the primary experiments, the selectivity of Carbon Paste electrode was tested against some cations.
Electrodes were utilized for solution (1.0×10-2_1.0×10-8M ) of the nitrate salt of the captions. Potential was measured and plotted versus the logarithm of the concentration of the cations. The data are shown in Table 1. According to data, La+3 cation shows a valid Nernstian response.
Optimization of CPEs
MWCNT-COOH was used as modifier in the manufacture of CPE. Besides, it can increase conductivity and improve dynamic range and reduce the response time of the electrode. To attain a suitable gradient, Nernstian response, and high selectivity, the percentage of Carbon Paste must be optimized. Then we optimized amount of MWCNTs and Graphite with one changing at the time method. Outcomes are shown in Table 2.
Table 2 represents the selected CPE optimal combination of 60% graphite powder, 25% paraffin, 5% SB-SalPr, and 10% MWCNT with a Nernstian response with a slope of 19.37 (mV/decade).
Calibration graph
As the results of the calibration curve show (Figure 2), the electrode exhibited a Nernstian response over a high Lanthanum ion concentration range of 10-2_10-8 M with slope of 19.37 mV per decade based on 3 repeat measurements.
The detection limit forLa+3 ion also gave the extrapolation of the linear area of the standard plot to the baseline potential, 5.21×10-9 (M).
Effect of pH
The pH influence was studied with 1.0×10-3and 1.0×10-4 mol L-1 La3+ in the range of 1-13.
Figure 3 demonstrated that potentiometric response remained constant in the pH range of 5-9.
The observed decrease in potential at pH>10 is attributed to the formation of some hydroxyl complex of La3+ ions. It was also observed that at lower pH values, the potentials increased. This was due to the prolongation of ionophore which affected a weak response to La3+ ions and strong response to H3O+ ions [27-28].
Dynamic response time
Response time is one of the important factors for each ion selective electrode (ISE). In order to measure dynamic response time, different concentrations of the La3+ from (10-2_10-8 mol L-1) were used. As it can be observed, the response time of electrode was less than 10 s (Figure 4).
Life time of the sensor
The life time of the electrode performance is: The length of time that the electrode will be the response to various concentration of the goal ion and slope is satisfactory. Each test was done using three electrodes 1 h during 10 weeks most ISEs had an average life time the ranging between 2 to 10 weeks. The Nernstian slope of the electrode decreased (from 19.37 to 12.56 mV per decade) after these intervals. Results are shown in Table 3.
Selectivity of the sensor
The potentiometric selectivity of a fabricated electrode is the response that the manufacture electrode gives to the target ion in the presence of other ions.
In this work, the selectivity coefficients were defined by the matched potential method (MPM). In MPM method, a known concentration activity ratio of primal cation (A) (1.0×10-7 M) and interfering cation (B) (1.0×10-5 M) in two separate experiments are added to the reference solution of primal cation (1.0×10-7 M); the measured potential matches the obtained before adding primal cation. Therefore, the MPM selectivity coefficient (KMPM) is the defined by the activity ratio of the primal ion (A) and the interfering ion (B) (KMPM=aA/aB) [29-37]. Results are shown in Table 4.
Analytical application
Modified electrode was applied to detect the end point of potentiometric titration of La3+ with EDTA. The solution of (1.0×10-3 M) EDTA was applied to titrate 25 mL of solution of (1.0×10-4 M) La3+. Following the addition of EDTA, the potential values decrease because of the formation of La3+ complex with EDTA formed leading to reduced concentration of free La3+ ions in solution. The relevant results are demonstrated in Figure 5.
This modified electrode was also used to specify the La3+ concentration in mixture of 2 and 3 various ions.
As is evident from Table 5, the recovery for La3+ ion was accepted in all mixtures.
As a result, we compared the new La-CPE with previous reports [37-39]. The results are shown in Table 6.
Conclusion
This project was designed to construct a CPE based on the N, N-Bus (salicyliden)-1, 3-propandiamine (SB-SalPr) ligand with the composition 5% ionophore, 60% graphite, 25% paraffin and 10% MWCNTs.
The fabricated electrode could be used for determineLa3+ ion and bestusagein range pH (5-9) and short response time <10 s. The concentration linear range of1.0×10-2_1.0×10-8 M La3+, a detection limit of 5.21×10-9 and with a Nernstian slope of 19.37 mV/decade were used. Therefore, this electrode can be used effectively to measure La3+ ions in real samples.
Acknowledgements
We thankfully acknowledge the support by the Islamic Azad University, Mashhad Branch.
Orcid:
Azam Hosseini Fahkrabad: http://orcid.org/0000-0002-0925-3309
Razieh Sanavi Khoshnood: http://orcid.org/0000-0001-5431-4839
Mohammad Reza Abedi: http://orcid.org/0000-0002-1262-4414
Mahmoud Ebrahimi: http://orcid.org/0000-0001-8637-9370
------------------------------------------------------------------------
How to cite this article: Azam Hosseini Fakhrabad, Razieh Sanavi Khoshnood*, Mohammad Reza Abedi, Mahmoud Ebrahimi. Fabrication a composite carbon paste electrodes (CPEs) modified with Multi-Wall Carbon Nano-Tubes (MWCNTs/N, N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chemical Communications, 2021, 3(9), 627-634. Link: http://www.echemcom.com/article_134775.html
------------------------------------------------------------------------
Copyright © 2021 by SPC (SamiPublishingCompany) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
