Document Type : Review Article
Authors
Pharmaceutical Chemistry Department, College of Pharmacy, University of Mosul, Mosul, Iraq
Abstract
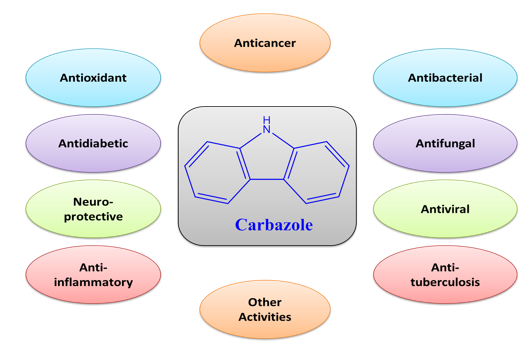
Carbazoles are a type of heterocyclic compound which has been shown to have a variety of biological properties, including anti-tumor, anti-bacterial, anti-fungal, anti-epileptic, anti-diabetic, anti-oxidative, anti-inflammatory, neuroprotective, and anticonvulsant effects. Carbazole can be considered by many medicinal chemists as a pharmacophoric nucleus because of its fused-ring structure with a highly conjugated system. This nucleus attracts the researchers’ attention for synthesizing a variety of bio-medically active compounds. Herein, an attempt is made to review the chemistry of this chemical nucleus and the biological potential of the carbazole-based derivatives.
Graphical Abstract
Keywords
Main Subjects
Introduction
Carbazole-derived products are a significant type of tricyclic moieties that are found broadly in nature [1]. They have been isolated from different sources, such as some genera of higher plants, blue-green algae, actinomycetes and filamentous fungi [2]. Carbazole chemistry and biology have attracted increasing interest since the description of the parent 9H-carbazole in the nineteenth century by Graebe and Glaser. Murrayanine, extracted from the stem bark of Murraya koenigii (Rutaceae), was discovered to have anti-bacterial properties, which found the evidence of pharmacotherapeutic activities associated to carbazole-derived products [3].
Carbazole-derived compounds have been reported in many scientific research papers to have various biomedicinal activities, including anti-histaminic, anti-oxidative, anti-microbial, analgesic, neuroprotective, anti-tumor, anti-epileptic, anti-inflammatory, and pancreatic-lipase inhibitory characteristics [1]. Due to the presence of a superior pharmacophoric moiety, carbazole-derived compounds are used as a starting material for the development of new medications and as an intermediary in the synthesis of several advanced molecules [4]. Many carbazole-based drugs are commercially available on the market, such as carvedilol, carprofen, carazostatin, ellipticine, ollivacine, datelliptium, alectinib, celiptium, and many others [1,4].
Chemistry of carbazole
Carbazole itself (9H-carbazole) (Figure 1) is indeed a heteroaromatic chemical molecule. Its framework is tricyclic, with two benzyl rings merged on either edge of a five-membered nitrogen-involving ring as pyrrole [5].
In addition to a potent π-conjugated system, the charge-transport and electronic properties of carbazole are desirable, and diverse operational groups can be easily presented into the architecturally constrictive carbazolyl ring moiety [7]. These structural characteristic features result in the wide and valuable applications of carbazole-based derivatives in the areas of medicine, chemistry in general, and medicinal chemistry, in particular [8]. Table 1 exhibits the general properties of carbazole.
Various biological potentials of carbazole-derived compounds
Anti-cancer potential
Cancer would be the second major cause of death globally, distinguished by the abnormal cells which can scatter to distant body sites, and can cause negative health consequences [9,6].
Three carbazole-based compounds have obtained marketing authorization as anti-cancer drugs. Their chemical structures are displayed in Figure 2. Ellipticine, chemically named 6H-pyrido[4,3-b]-5,11-dimethylcarbazole, is a naturally occurring alkaloid that was extracted from the leaves of Ochrosia elliptica more than 60 years ago and is regarded as the first carbazole-based product’s initial lead compound. The second authenticated drug, alectinib, has a 5H-benzo[b]-carbazol-11(6H)-one scaffold and was approved by the FDA in 2015. The last drug was approved in 2017 by the FDA and is named midostaurin [9]. These three FDA-approved drugs have the capacity to converse with DNA and down-regulating of DNA topoisomerase-II. They also establish covalent Dimers as a result of cytochromes P450 and peroxidases oxidizing them [6].
Liu and his colleagues created new N-acyl and N-alkyl functionalized carbazole-sulfonamide hybrids and tested their own profitability inhibition effect in Bel-7402, HepG2, and MCF-7 cell lines. Hybrids with shortened alkyl chains and smaller functional groups at the N-position of the sulfonamide moiety were more effective as cytotoxic agents than those with longer alkyl chains and larger functional groups, according to the structure-activity relationships. The sodium phosphate of 6-(N-(2,6-dimethoxypyridin-3-yl)sulfamoyl)-9-methyl-9H-2-carbazolly (Figure 3) indicated the much more powerful and effective profitability inhibitory action, the least normal tissue harm effects, and the best pharmacokinetic and pharmacodynamic of the synthesized hybrids [10].
Bondock and his colleagues have synthesized, characterized, and mechanistically explored a new group of carbazole-condensed heterocyclic compounds. Pyrazo[3,4-d]-[1,2,3] triazines, pyrazolo[1,5-a] pyrimidines, and imidazo[1,2-b] pyrazoles are structural precursors of this group. Seven members of the investigated group had good-to-moderate viability inhibitory activity versus three human cancerous line cells, involving HepG-2, HCT-116, and MCF-7, and one normal human cell line, abbreviated as REP1. The best compound, with a chemical structure displayed in Figure 4, can minimize the development of cancer cell lines tested and show no harmful toxic effects on standard cell lines [11].
In another study, Huang and co-workers designed, synthesized, and biologically evaluated two series of the novel carbazole-based amides and carbazole-based acylhydrazones. The findings of this study indicated that some of these synthesized conjugates showed promising viability inhibitory activity against HepG2 and A875 cancerous cell lines. As illustrated in Figure 5, the carbazole-based acylhydrazones containing the structural core of 1,3-benzodioxole had significant selectivity between the tested cancerous and normal cells. Based on the obtained results, the authors concluded that this structural core may represent a lead compound for synthesizing more effective cytotoxic agents [12].
Anti-bacterial Potential
The introduction of drug-resistant bacteria had already negatively affected the efficacy of a number of commonly used anti-bacterial prescription drugs [13]. As a result, several efforts have been made to discover new anti-bacterial agents [7]. Many medicinally active compounds, which occur naturally, contain the carbazole ring. For example, the carbazomycins are a novel class of antibiotics with a carbazole framework [14]. Carbazomycins A and B, with chemical structures illustrated in Figure 6, have been demonstrated to inhibit 5-lipoxygenase and exhibit anti-bacterial and anti-fungal properties, making carbazole-based compounds a promising lead for further biological research [15,16].
It has been discovered that carbazole-based molecules may have numerous anti-bacterial pathways. One possibility is that these molecules boost membrane permeability by hindering specific enzymatic mechanisms, enabling extra reactive oxygen species to pass through the macrophages of the human immune response [17]. The second proposed inhibitory mechanism of action is the interaction of carbazole-based compounds by non-covalent bonding with bacterial DNA gyrase [18].
Dabrovolskas and co-workers synthesized various mono-substituted, di-substituted, and tri-substituted carbazole-based products utilizing various substituents, including halogens, isonitrile, and alkyl-containing functional groups, as illustrated in Figure 7. This team’s work evaluated the anti-bacterial activity of the synthesized carbazoles against Bacillus subtilis and Escherichia coli using a well-defined method of disk-diffusion technique. The findings indicated that six out of seven tested carbazole-based compounds had anti-bacterial activity against tested strains, and the compounds functionalized at C-6 and C-3 positions showed better anti-bacterial activity than the carbazoles substituted at C-2 and C-7 positions. Furthermore, gram-positive bacteria are inhibited more effectively by the synthesized carbazoles with iodide substitutions, while gram-negative bacteria are suppressed more effectively by those functionalized with bromide substitutes [19].
In another study, Shaikh and his colleagues developed novel carbazole-based conjugates and tested their anti-microbial activity in vitro against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, and four fungal strains. The nature of the substituents on the carbazole synthetic core had a major impact on the anti-microbial properties of carbazole-based conjugates, according to the research results. The bioactivity of the compounds was significantly influenced by nitrogen-containing hetero-aromatic structures on the carbazole nucleus. Halides and their structural and functional groups (CF, Cl, Br, I, and F) lowered the anti-microbial activity profile by removing electrons. Figure 8 displays the molecular structure of the most effective carbazole-based conjugate, which has a good anti-microbial activity and it can be considered a lead compound [20].
In the same context, novel carbazole-based compounds holding a thiosemicarbazide, dihydrotriazine, semicarbazide, or isonicotinic moiety have been designed and synthesized by Xue and his co-workers. The anti-bacterial activity of the synthesized carbazoles against Staphylococcus aureus, Streptococcus mutans, Methicillin-resistant Staphylococcus aureus and Escherichia coli was evaluated. The majority of the acquired carbazoles indicated significant inhibitory impact versus different investigated bacterial strains, including one multidrug-resistant clinical isolate. The results afforded from studying the structure-activity relationship and in silico docking revealed that the carbazole functionalized with a dihydrotriazine group, as illustrated in Figure 9, had the best antimicrobial potency and least toxicity among the synthesized carbazoles [21].
Anti-fungal Potential
Recently, the resistance to anti-fungal agents has become a health concern. Given the existence of newly developed anti-fungal and curative schemes, insidious fungal infection mortality and morbidity keep rising [20]. Systemic fungal infections are life-threatening, mainly if they occur in immune-competent and immune-compromised humans, such as patients with COVID-19, patients with AIDS, and immunosuppressant-organ transplant patients [22]. One medicinally active substance which occurs in nature is carbazomycin G, with the chemical structure displayed in Figure 10, having a unique quinolone moiety and showing antifungal activity against Trichophyton species [15].
Shaikh and his colleagues, as mentioned above, synthesized new carbazole conjugates and evaluated their antimicrobial activities in vitro against Aspergillus niger, Cryptococcus tropicalis, Cryptococcus neoformans, and Candida albicans. The most active hybrid, with the chemical structure illustrated in Figure 8, exhibited prominent anti-microbial activity against Cryptococcus neoformans [20].
Novel carbazole-N–hydrazinoacetyl conjugates were created by Hegden and colleagues. In addition to docking studies, the anti-fungal activity of these compounds was investigated using griseofulvin as a classic antifungal drug against Candida albicans and Aspergillus niger. Once evaluated by comparing to the molecular docking of the reference drug, the conjugate, chemically named 1-carbazole-9-yl-4,5-diphenyl-1H-imidazole-1-yl-2-(2-cyano phenyl)-amino)-ethanone), and its chemical composition displayed in Figure 11, demonstrated increased docking marks. In addition, the mentioned conjugate had greater anti-fungal properties in vitro against the test pathogenic fungi than the other synthesized conjugates as well as the standard [23].
Park and his colleagues investigated the anti-fungal potential of 31 carbazole-based compounds on the invasion of the pathogenic fungus named Candida albicans and their safety profile towards mammalian cells compared with fluconazole. The findings gathered from this evaluation suggest that the compounds, named molecule-C and molecule-B, with the chemical structures displayed in Figure 12, are promising candidates for suppressing the invasion of Candida albicans by inhibiting hyphal formation, as hyphal morphogenesis is important for the pathogenesis of this infective fungus [24].
Anti-viral Potential
Aside from the chickenpox, common cold virus, gastroenteritis (stomach flu), herpes, hepatitis, and human immunodeficiency virus (HIV, the causative agent of AIDS), viruses are the initiators of a large number of widespread infectious diseases, such as the pandemic H1N1 influenza-virus, Ebola virus (EBOV), novel COVID-19. Viral infections can result in serious and potentially fatal complications, and they are probably the cause of much more than 60% of ailments in developed nations [25,26].
Saturnino and his co-workers created many chlorinated derivatives of 1,4-dimethyl-9H-carbazole and studied their inhibitory activity against HIV-1. The nitro-derivative had significant activity, indicating that the chlorine position on the carbazole scaffold, as displayed in Figure 13, could be responsible for its anti-viral activity, and it can be enhanced by the concomitant presence of an electro-attractor group, like the nitro functional group [27].
Rassias et al. discovered the novel carbazole-based derivatives of the prototypic chemical structure illustrated in Figure 14. These derivatives were capable of inhibiting cell-active ZIKV protease, the enzyme with submicromolar potencies and significant cellular activities, generated by rational design. Bis- and mono-amidine compounds, as well as carbazole N-substitution, were indicated to be tolerable in terms of potency and toxicity [28].
A team led by Spizzichino and his colleagues synthesized various derivatives having a carbazoyl-aromatic urea core framework, and the ducking studies applied to these derivatives using the ZIKV NS5-MTase (ZIKV N-terminal Methyltransferase domain) indicated the potential impact of the carbazole ring on the anti-viral activity by its substitution with different aromatic and hetero-aromatic moieties. As exhibited in Figure 15, the substitution of N-benzyl or N-phenylethyl on the carbazole helps these compounds bind to the viral proteins [29].
Anti-tuberculosis Potential
Tuberculosis will have killed 3 million people by 2020. This highly infectious disease, abbreviated as TB, is the world’s 13th leading cause of death and, after COVID-19, the second leading infectious killer. Multidrug-resistant TB is a serious public health problem, and new medicines are desperately needed [30].
In their work, Sellamuthu and co-workers analyzed the structure-activity relationship of many carbazole-based compounds as anti-tubercular agents. The researchers discovered that the substitution on a phenyl moiety, rather than the N-substitution on the carbazole chemical nucleus, is required for activity. The conversion of the phenyl moiety to other fused ring system, bi-cyclic or tri-cyclic, rather than carbazole, has not improved the activity, indicating that the carbazole chemical nucleus was essential for the action [30].
A team led by Schmidt synthesized olivacine and pyrido derivatives. The researchers found that the compound named 9-methoxyolivacine, with the chemical structure illustrated in Figure 16, was the most active agent, with a good safety profile towards the tested mammalian cell line. These results prove that the pyrido derivatives of olivacine are potential applicants for the incoming exploration for new carbazole-based anti-tuberculosis drugs [31].
Surineni and his colleagues have synthesized many N-methyl derivatives of dibenzothiophene, dibenzofuran, and carbazole tethered to thiazolyl cinnamamide. The findings indicated that N-methylcarbazole derived 4-bromofunctionalized derivative, with the chemical structure illustrated in Figure 17, had a high activity profile and it could be the best applicant for further development [32].
Anti-inflammatory Potential
Inflammation is a physiological model which defines the body’s reaction to a variety of signals and has been associated with a number of abnormalities, including psoriasis, arthritis, and asthma, that require long-term or recurring treatment. The rate-limiting enzyme of the prostanoid biogenesis for producing thromboxane A2, prostacyclin, andprostaglandins is the cyclooxygenase (COX) enzyme family [33].
There are two isoforms of the cyclooxygenase enzyme. The first is cyclooxygenase-1 (COX-1) which produces pro-aggregatory thromboxanes in blood platelets and cytoprotective prostaglandins in the GIT system. It would be therapeutic to suppress the enzymatic activity of the second isoform, cyclooxygenase-2 (COX-2), which is involved in the bad consequences of inflammation and pain [33].
The research team led by Pattanashetty has synthesized two new series of carbazole-based compounds. These are N-phenylacetamide-functionalized carbazoles and methyl-2-(6-chloro-9-(2-oxo-2-(phenylamino)ethyl)-9H-carbazol-2-yl)propanoates. The anti-inflammatory activity of the members of these two series was assessed using egg albumin denaturation method. Some of the compounds, as that with the chemical structure displayed in Figure 18, indicated excellent anti-inflammatory activity [34].
Liu et al. have isolated and identified twelve prenylated carbazole-based alkaloids from the stems and leaves of Clausena vestita, a Chinese endemic plant. The anti-inflammatory effect of these carbazole-based natural products on mouse macrophage RAW 264.7 cells was assessed in vitro. The results confirmed that three natural alkaloidal products, with the chemical structures illustrated in Figure 19, possess remarkable anti-inflammatory effects and may consider as structural templates for the potent anti-inflammatory candidate drugs development [35].
Neuroprotective potential
Neuro-protection involves the pathways able to preserve the central nervous system (CNS) from the damage induced by chronic degenerative illnesses (e.g., Parkinson’s and Alzheimer’s diseases) or acute injury (e.g., trauma and stroke) [36].
A study conducted by Bachurin and his research team involved the design of multi-target therapeutic agents for managing neurodegenerative diseases. These agents were prepared by conjugating aminoadamantane with various carbazole-based moieties. The authors discovered that these agents can protect nerve cells from death under calcium overload conditions, block NMDA (N-methyl-D-aspartate) receptors, and stabilize microtubules [37]. Figure 20 represents the chemical structure of the most effective carbazole-based compound that was synthesized by Bachurin et al.
Elmabruk et al. have designed a panel of derivatives based on their structures on carbazole as multi-functional agents for managing Parkinson’s disease. The compounds, with the core chemical structure illustrated in Figure 21, were synthesized by incorporating the aminotetralin or bioisosteric equivalent agonist head group with carbazole. The tested derivatives showed vigorous agonist activity at both dopaminergic receptors 2 and D3 and could reduce oxidative stress induced by the neurotoxin 6-hydroxydopamine. These observations make them promising multi-functional molecules for managing Parkinson’s disease and should be further investigated [38].
A research team led by Liu has separated 16 carbazole-based alkaloids, including six new ones from the fruits named Clausena lansium and established their molecular structures based on a comprehensive spectroscopic method. In vitro study for evaluating the neuroprotective impact of the separated alkaloids on 6-hydroxydopamine-stimulated apoptosis in human neuroblastoma was conducted and indicated that these compounds, specifically those with chemical structures illustrated in Figure 22, had remarkable neuroprotective cells and could be crucial for the discovery of new agents for the prevention and treatment of Parkinson’s disease [39].
Anti-diabetic potential
Diabetes mellitus (DM) is a prolonged condition which is characterized by elevated blood glucose levels and is closely correlated with dyslipidemia. It is caused by a combination of genetic and environmental factors. Diabetes becomes a worldwide problem, with over 90% of diabetics diagnosed with diabetes type-2, a serious condition with elevated mortality rates and handicap rates. There are numerous oral anti-diabetic promoters available today, although they have a number of side effects, particularly when patients take them for an extended period of time. As a result, there seems to be an ongoing need to explore and build innovative anti-diabetic drugs [40].
Iqbal and his colleagues created a new class of carbazole-1,2,3-triazole hybrids and tested their in vitro α-glucosidase inhibitory activity against the standard drug acarbose. Two of the synthesized compounds, with the chemical structures illustrated in Figure 23, indicated better inhibitory activity, meaning that by the chemical modifications of carbazole-type scaffolds, bioactivities can be tuned for possible applications [40].
In a separate study, Eseyin et al. synthesized four alkyl- (methyl-, ethyl-, propyl-, and butyl-) carbazoles and investigated their in vitro inhibition of α-amylase and α-glucosidase. These alkylated carbazoles show significant inhibitory effects on α-amylase, and this inhibitory effect is directly proportional to the chain length of the alkyl group. The inhibitory effect of these carbazoles on α-glucosidase was found to be insignificant [38].
Adib and his colleagues designed, synthesized, and screened twenty-three fused carbazole-imidazole conjugates as novel blockers of α-glucosidase compared to acarbose. The conjugates, which have the fundamental chemical composition displayed in Figure 24, have such a higher inhibition activity on α-glucosidase than the regular agent acarbose, and some of them have had an inhibition effect 8-10 times that of the regular agent [36].
Anti-oxidant potential
Large amounts of free radicals and reactive oxygen species, such as reactive hydroxyl, hydrogen peroxide, and several other free radicals, can cause health problems in aerobic organisms. These disorders include drug-associated toxicity, carcinogenesis, atherogenesis, inflammation, and aging. Anti-oxidants are currently being investigated as potential therapies to consider these disorders [29].
A new alkaloid containing 3-hydroxycarbazole named carazostatin was isolated in 1989 from Streptomyces chromofuscus and considered as a free radicals-terminating agent. This natural carbazole-based alkaloid, with the chemical structure depicted in Figure 25, exhibits strong inhibitory activity against lipid peroxidation induced by free radicals and illustrates a powerful free-radicals-terminating potential in liposomal membranes than vitamin E [32].
In a medicinal chemistry study, Karaaslan and his colleagues synthesized, characterized, and studied the in vitro anti-oxidant activity of a series of 9-ethyl-9H-carbazole-hydrazone conjugates. Their chemical core structure is illustrated in Figure 26. The results indicated that the conjugates with a halogen-substituted aromatic moiety had a reducing potential against non-fluorescent 2',7'-dichlorofluorescein oxidation, especially the fluorinated ones. While the meta-substituted conjugates were found to have higher activity against 2,2'-diphenyl-1-picrylhydrazyl (DDPH) radicals [12].
Bordei et al. studied the in vitro anti-oxidant activity of some new 6-chloro-9H-carbazol-based compounds employing scavenger activity towards DPPH and ABTS•+ (2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid)) free radicals. The chemical structures of the carbazole-based compounds having significant antioxidant capacity and an adequate safety profile are displayed in Figure 27. When compared to the DPPH assay, the scavenger capacity towards ABTS•+ free radical was greater [33].
In a separate study, Serdaroğlu and co-workers synthesized various carbazole-based compounds and studied their anti-oxidant potential by using the CUPRAC (CUPric Reducing Anti-oxidant Capacity) methodology. The compound with the chemical structure illustrated in Figure 28, and named ortho-acetyl oxime derivative of (E)-2,3-dihydro-1H-carbazol-4-(9H)-one, showed the best antioxidant activity [37].
Other Bio-Potentials
Some compounds with a carbazole moiety were found to have anti-convulsant activity. Low-dose hydrogen sulfide (H2S) has been shown to have anti-epileptic and defensive effects on the nervous system, but administering H2S gas to stop seizures is difficult. Zhu and colleagues created a new carbazole-based H2S supplier with the structural formula indicated in Figure 29, which supplied a low and stable H2S source. When the H2S supplier was infused into the lateral ventricle of a rat model of sophisticated seizures, the inhibition of seizures was ascertained in the nervous system. [55-57]. The most important advantage of the synthesized donor is the exertion of an anti-epileptic effect similar to that of traditional H2S donors but without significant biological toxicity [39].
Several natural and synthetic carbazole-based derivatives have been found to have anti-malarial activity. Kadnor and his colleague synthesized two series of carbazole-based 1,4-benzothiazepine and -based pyrazoline derivatives. The core chemical structures of these two series are illustrated in Figure 30. The researchers [28-36] studied the anti-microbial and anti-malarial activities of these carbazoles. The anti-malarial assessment demonstrates that action is reduced as the electronegativity of substituents connected to aromatic rings is reduced, with the carbazole without the need for a functional group in the phenyl ring having the least activity against various fungal, bacterial, and anti-malarial strains [20].
Conclusion
Carbazole is an important heterocyclic molecule which serves as a unique template for a variety of biological functions. Numerous carbazole-based products and compounds have been produced and studied with varied biological activity throughout the last decade. This article is a comprehensive review of the most recently isolated and synthesized carbazoles with various biological potentials that have been reported. More research on carbazole as a biomedical pharmacophore is needed, because it’s a promising class of drugs for treating a variety of diseases.
Acknowledgements
The authors are very grateful to the University of Mosul/College of Pharmacy for their provided facilities, which helped to improve the quality of this work.
Ethical issues
The scientific committee of the Pharmaceutical Chemistry Department was approved this work
Competing interests
We have no conflicts of interest to disclose.
Authors’ contributions
All authors contributed toward data analysis, drafting and revising the paper and agreed to responsible for all the aspects of this work.
Orcid:
Seema Mahmood Kasim: https://www.orcid.org/0000-0002-2061-8559
Baraa Moulood Al‐Dabbagh: https://www.orcid.org/0000-0002-0318-9625
Yasser Fakri Mustafa: https://www.orcid.org/0000-0002-0926-7428
------------------------------------------------------------------------------------
How to cite this article: Seema Mahmood Kasim, Baraa Moulood Al-Dabbagh, Yasser Fakri Mustafa*. A review on the biological potentials of carbazole and its derived products Eurasian Chemical Communications, 2022, 4(6), 495-512. Link: http://www.echemcom.com/article_147133.html
------------------------------------------------------------------------------------
Copyright © 2022 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
