Document Type : Original Research Article
Authors
Department of Chemistry, Chemical Institute of Kazan Federal University, Russia
Abstract
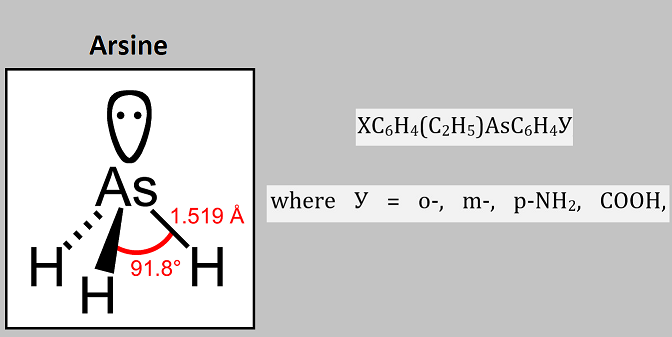
The experimental possibility of splitting asymmetric derivatives of three-and four-coordinated arsenic into optically active antipodes had for a long time remained unattainable for many well-known chemists, including Meisenheimer (Germany), Kamai (Russia), etc. When a racemic mixture of chiral arsines is cleaved with optically active components, a functional group (acidic or basic) is used that is contained in one of the fragments bound to the arsenic atom. Chiral arsines containing a carboxyl group are synthesized by oxidation of the corresponding fragments. Based on the results, to develop effective methods for the synthesis of tertiary arsines containing functional groups XC6H4(C2H5)AsC6H4У, where У=о-, m-, p-NH2, COOH, the reactivity of ethylarylarsine chlorides with organometallic compounds should be considered.
Graphical Abstract
Keywords
- Alkyldiarylarsines
- ethylarylarsine chlorides
- asymmetric
- secondary
- tertiary arsines
- carboxyphenylarsines
- chiral arsines
Main Subjects