Document Type : Original Research Article
Authors
1 Department of Biochemistry, College of Medicine, University of Missan, Missan, Iraq
2 Department of chemistry, College of Science, University of Baghdad, Baghdad, Iraq
Abstract
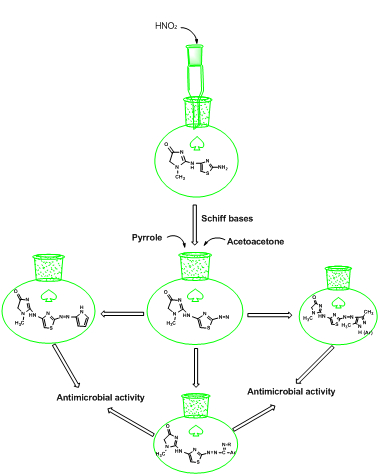
The present work include synthesized new 2-amino-4-subs. thiozole(1) from reaction of 2-N-chloro acetamido Creatinine with thiourea. Compound (1) was treated with sodium nitrate and hydrochloric acid in (0-5ºC) to form diazonium salt (2), then diazonium salt reacted with acetylacetone and hydrazine, phenyl hydrazine and 2,4-dinitrophenyl hydrazine to give pyrazole ring (4-6). On the other hand, diazonium salt was react with pyrrole in the presence of glacial acetic acid to form compound (7) and with different Schiff bases to produce compounds (8-9). Prepared compounds were measured by IR and melting point and some of them by 1HNMR and their biological activity was studied.
Graphical Abstract
Keywords
Main Subjects
Introduction
Heterocyclic compounds constitute a key component in a lot of natural products, to name a few; vitamins, hormones, alkaloids, a wide range of antibiotics, pharmaceutical products, herbicides, anti-aging medicines, and plenty other industrial products of high importance (different types of dyes, corrosion inhibitors, stabilizing agents, sensitizers, etc.) [1]. Aryl diazonium salts are easily prepared, common, and highly applicable intermediates in synthetic organic chemistry because of their high reactivity and varied reactions. They are synthesized starting from primary aromatic amines by diazotization and coupling with aromatics like phenols (or primary aromatic amines). Azo dyes are industrially very important for technical purposes. Azo compounds have many applications such as their use as antioxidants, polymeric biodegradable pro-drugs, and many of them are used in the food, cosmetics, and drug industry as synthetic colorants [2]. Pyrazoles heterocyclic compound has a five-membered ring containing two nitrogen atoms prepared by many methods, one of these methods is the condensation of hydrazine or substituted hydrazine with α, β- unsaturated carbonyl compounds [3]. Azo compounds are well known for their medicinal importance and are recognized for their applications as antifungal, antidiabetics, antineoplastics, anti-inflammatory, antiseptic [4]–[6], and other useful chemotherapeutic agents. They are involved in many biological reactions such as inhibition of DNA, RNA, carcinogenesis, protein synthesis, and nitrogen fixation [7]. Azo compounds are valuable in the medicinal and pharmaceutical fields [8].
Material and methods
In this research, all starting material and solvents that used obtained from (Sigma-Aldrich, and Fluke Company, Germany). FT-IR spectra (KBr disc) were recorded with Affinity-1 Shimadzu as an FT-IR spectrometer using KBr pellets. 1HNMR spectra scanned on Bruker Spectro spin ultrashield magnets 400 MHz instruments.
Synthesis of 1-methyl-2-(2-amino-thiazole-4-yl) amino-4-oxo-4,5-dihydro imidazoline[9](1).
A mixture of thiourea (0.01 mol, 0.76 g) and (0.005 mol, 0.94 g) of 2-N-chloro acetamido Creatinine dissolved in 100 mL of CH3OH in the flask and refluxed for 3–4 hr. The initial product cooled then poured into cold H2O. The solid separation was collected by filtration. The residue obtained was dried and purified by using C2H5OH.
Synthesis of 1-methyl-2-(2-diazenyl-2,4-dioxo-3-pentane--thiazol-3-yl)amino-4-oxo-4,5-dihydro imidazoline[10](2).
Compound [1] (0.21 g, 0.001 mmol) was dissolved in 2 mL conc. HCl. Cooled at 0 °C, then NaNO2 (0.07 g, 0.001 mol) in (5 mL) of H2O was added dropwise with stirring for 30 min. in an ice bath at 0-5 °C, then acetylacetone (0.1 g,0.001 mol), CH3COONa(0.16 g, 0.002 mmol) in CH3CH2OH (5 mL) was added drop by drop. The mixture was then stirred for (30 min.). The product was purified by methanol.
Synthesis of 1-methyl-2-[(2-(3,5-dimethyl-pyrazol-4-yl)diazinyl] amino-4-oxo-4,5-dihydro imidazoline derivatives[11](4-6).
NH2-NH2 derivatives (0.006 mol) were added to compound [3] (0.19 g, 0.006 mol) in (10 mL) EtOH. The mixture was stirred and refluxed for (10-12 hour), then the solvent was evaporated and the product was washed with H2O then (C2H5)2O.
Synthesis of 1-methyl-2-(2-amino-thiazole-4-yl)amino-4-oxo4,5-dihydro imidazoline[12](7).
A (0.01 mole) of pyrrole was dissolved in ethanol (10 mL) and CH3COONa (0.3 g) was added and the mixture cooled and stirred. Cold solution of ArN2Cl salt of compound (1) was then added dropwise for 1 hr at (0-5 °C) and the mixture kept in a cold place for 3 hr and then poured into ice H2O.
Synthesis of 1-methyl-2-[(2-(4-subs.)benzylidene] amino-1-methyl-4-oxo-4,5-dihydro imidazoline [13](8-9).
A (0.01 mol) solution of compound 1 was dissolved in 2 mL eq. HCl. It was cooled and 0.7 g of sodium nitrate was slowly added, 2-N-arylidene amino creatinine (0.01 mol) was dissolved in 10mL C5H5N and 0.3 g of sodium acetate was added to the mixture and then the mixture was stirred and cooled in the cold place. cold solution of ArN2Cl salt of compound (1) was added dropwise for 1hr at (0-5 °C). The reaction mixture was kept in ice-bath for 3hr. The resulting dark-color mass was filtered, washed with H2O until C5H5N removed. The product was purified from absolute CH3CH2OH.
Biological activity [14].
By using the agar plate diffusion method, the prepared compounds screened in vitro for two types of bacteria staphylococcus (gram-positive) and E-coli (gram-negative). Inhibition zone of bacterial growth show in Table 3.
Results and discussion
In this research, synthesis of azo-compounds from 2-N-chloro acetamido creatinine was done, as shown in the Scheme 1. The azo-derivatives of creatinine measured by IR and some derivatives by 1HNMR.
SCHEME 1 Syntheses new azo-derivatives from creatinine
Synthesized compounds (1-9) were detected by spectral (FTIR & 1H-NMR). In the compound (1) 1697 cm-1 due to amide group [15]. The 1HNMR of compound (1) δppm in DMSO-d6 solvent showed singlet signal at δ(1.15) ppm due to (-CH3) protons, singlet signal at δ(2.48) ppm due to (C=O-CH2-N-CH3) proton, singlet signal at δ(3.05) ppm due to (CH-S-thiazole ring) singlet signal at δ(7.13) ppm due to (creatinine ring-NH- thiazole ring), and a single signal at δ(4.13) ppm due to NH2 protons of thiazole ring. The presence of the band in compound (3) at 1546 cm-1 indicates the formation N=N and 1740 cm-1 that refer to C=O of diketone. In pyrazole compounds (3-5) the absorption band in compound (5), and 1HNMR for compounds 5,6, and 7 are show in Table 2.
Conclusion
The prepared compounds were measured by using (FT-IR and 1HNMR). The biological studies of the new azo compounds showed inhibitory effects on two types of bacteria, Staphylococcus aureus and Escherichia coli. Regarding Staphylococcus aureus, compounds No. 6 and 9 showed moderate inhibition, while compounds No. 5,7 and 8 exhibited slight inhibitory effect. On the other hand, the growth of E Coli was highly inhibited by the compounds No. 6 and 9 and moderately inhibited by compounds No. 7 and 8 and only slightly inhibited by the compound No. 5. In conclusion, the results of the current study demonstrated that these prepared compounds have good efficacy against the tested bacteria.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship at Baghdad University College of Science, and I want to thank everyone who helped me to complete this research.
Orcid:
Raad M. Muhiebes:
https://www.orcid.org/0000-0003-0181-5837
|
How to cite this article: Raad M. Muhiebes* , Entesar O. Al-Tamimi. Synthesis of new heterocyclic containing azo group from 2-N-chloro acetamido creatinine and studying their biological activity. Eurasian Chemical Communications, 2021, 3(6), 401-405. Link: http://www.echemcom.com/article_130622.html |
Copyright © 2021 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.