Document Type : Original Research Article
Authors
Petroleum Research and Development Center (PRDC), Ministry of Oil, Baghdad, Iraq
Abstract
The viscosity of lubrication oil is the important property to evaluate the behavior of lubrication activity of oil. Viscosity index VI is the standard indicator of effecting temperature on viscosity of lubricating oil.
Viscosity index improvers are the chemicals that added to base lubricating oil produced from refineries to make oil stable against temperature elevation during internal combustion engine operation.
In this work, special viscosity index improver was prepared by blending of DQC (that synthesized depending on Ethylene Diamine Tetra Acetic acid original compound) with polyoxyethylene (p 20) in presence of mystelene as solvent. The prepared viscosity index improver was added to base lubricating oil grade SN-500 produced from Al – Dorah refinery with different weight ratios, adding 7.8% of viscosity index improver to SN-500 base oil improved VI by 14 degrees without effect on other properties of oil.
Graphical Abstract
Keywords
Main Subjects
Introduction
The growth of lubrication technology has largely depended on the development within petroleum industry in the refining and utilities of crude mineral oil [1].
The development in chemical engineering technology in the last years made it possible not only to separate these fractions of mineral oil useful as lubricants, but also provide means of modifying of reinforcing their properties as lubricants by addition of other chemical structures, usually in relatively small amounts, which exert powerful physical or chemical effects on these properties [2,3].
Lubrication is the process, or technique employed to reduce wear of one or both surfaces in close proximity, and moving relative to each other, by interposing a substance called lubricant between the surfaces to carry or to help carry the load (pressure generated) between the opposing surfaces [4].
A lubricant is a substance introduced to reduce friction between moving surfaces. It may also have the function of transporting foreign particles. The property of reducing friction is known as lubricity.
A good lubricant possesses the following characteristics:
- High boiling point;
- low freezing point;
- high viscosity index;
- thermal stability;
- corrosion prevention; and,
- high resistance to oxidation.
One of the single largest applications for lubricants, in the form of motor oil, is protecting the internal combustion engines in motor vehicles and powered equipment [5,6].
Motor oil is a lubricant used in internal combustion engines. In engines, there are parts which move against each other causing friction which wastes otherwise useful power by converting the energy to heat. Contact between moving surfaces also wears away those parts, which could lead to lower efficiency and degradation of the engine. This increases fuel consumption, decreases power output and can, in extreme cases, lead to engine failure [7,8].
Viscosity of lubricants
Probably the most important single property of lubricating oil is its viscosity. This factor plays a role in the formation of lubricating films under both thick and thin film conditions. Viscosity affects heat generation in bearings, cylinders, and gears; it governs the sealing effect of the oil and the rate of consumption or loss; and it determines the ease with which machines may be started under cold conditions. For any piece of equipment, the first essential for satisfactory results is to use an oil of proper viscosity to meet the operating conditions [9].
Lubricants work because they have viscosity; viscosity is the most misunderstood aspect of oil and yet it is the most important. It is the property of a fluid that causes it to resist flow, which mechanically is the ratio of shear stress to shear rate. Viscosity may be visualized as a result of physical interaction of molecules when subjected to flow.
The temperature dependence of liquid viscosity is the phenomenon by which liquid viscosity tends to decrease (or, alternatively, its fluidity tends to increase) as its temperature increases.
Viscosity is a property of a liquid related to its state transition temperatures. Let's first review the different forms or states that any substance like oil can take [10].
At any temperature and pressure, most substances can exist in one of three states: Solid, liquid, or gas. The state a substance takes depends on its temperature and the pressure to which it is being subjected. At an atomic level, the two main forces at work that determine whether a substance is solid, liquid, or gas are: the kinetic (heat) energy of the atoms, and the attraction between the atoms.
Viscosity index (VI)
Viscosity index is a number assigned to lubricants that describes how much their viscosity changes with temperature change. A lubricant with a higher viscosity index has demonstrated lower amounts of viscosity change. Lubricants with a high index give better protection and efficiency than their low index counterparts. High viscosity index lubricants are more likely to retain correct viscosity for the specific application, because they resist thickening in cold temperature operation or thinning in high temperature operation [11].
Viscosity modifiers
Viscosity of oils sharply decreases at high temperatures. Low viscosity causes decrease of the oil lubrication ability. Viscosity index improvers keep the viscosity at acceptable levels, which provide stable oil film even at increased temperatures. Viscosity improvers are widely used in multi grade oils, viscosity of which is specified at both high and low temperature.
Viscosity improvers are long chain, high molecular weight polymers that function by causing the relative viscosity of an oil to increase more at high temperatures than at low temperatures. Generally this result is due to a change in the polymer’s physical configuration with increasing temperature of the mixture. It is postulated that in cold oil the molecules of the polymer adopt a coiled form so that their effect on viscosity is minimized. In hot oil, the molecules tend to straighten out, and the interaction between these long molecules and the oil produces a proportionally greater thickening effect. A Viscosity improver does not just make the engine oil thicker, it acts to even out the logarithmic relationship between viscosity and temperature; as the temp increases a little, the fluid thins out a huge amount.
The relationship between temperature and viscosity for pure mineral oils is well known. A VI improver is typically a high-molecular weight polymer which has the desired characteristic of swelling in size as the temperature increases. This increase in molecular size increases the viscosity due to that polymer, which offsets the decrease in viscosity of the base oil. In this way, the VI of oils can be increased by use of these polymers.
In general, VI improvers are not as stable as the base oils they are added to, and have less resistance to oxidation or degradation due to shear than does the base oil. Therefore, between two oils with the same VI, the one with the lower amount of VI improver would be more thermally stable and suffer less viscosity loss, all else being equal [12].
Experimental
The experimental section included three steps:
- Preparing tetra amino compound (DQC).
- Mixing amino compound with polymer P20 Poly sorbate.
- Adding prepared mixture (tetra amino compound + polymer) to basic oil.
Preparation and addition
Preparing tetra amino compound (DQC)
The (DQC) compound was prepared through
two steps:
A- First Step: preparation of Fatty alkyl halide:
It took place through mixing of hexadecanol (CH3(CH2)15OH, mp=54-56 ° C) with hydrobromic acid (48%) (0.05 mole) then refluxing for seven hours with the presence of sulfuric acid (1.62 mL) as a catalyst , after reflux is finished, the mixture was cooled and poured into a separating funnel to separate the aqueous layer from the organic layer. After separation, dry hot methanol was used several times to extract non-reacted hexadecanol, the product was dried using suitable amount of magnesium sulfate for hours , then filtration was done. The yield was 80% as indicated in equation (1), the drops of sulfuric acid that was used as a catalyst was removed through being in aqueous layer.
B- Second Step: reaction of Fatty alkyl halide with EDTA.
In the reaction flask, 0.01 mole of EDTA (ethylenediamine tetraacetic acid) was mixed with 0.02 mole of the fatty alkyl halide prepared in the first step to guarantee the orientation of alkyl halide towards the two nitrogen atoms of EDTA was then refluxed for 24 hours in the presence of ethanol as a reaction media. Alcohol was separated using rotatory evaporator. The product was purified and dried to get a semi solid fatty nature powder; to get DQC as shown in Equation (2).
Mixing amino compound with polymer polysorbate P20
In this step, 0.4 g of tetra amino compound that was prepared in the first step was added to 6.6 g of polymeric substance (polyoxyethylene), the mixture was heated at 70 °C for two hours along with continuous mixing, after that the mixture was left to cool then its stability was checked during 72 hours
Using the prepared mixture (amino compound + polymer) to improve oil viscosity
In this step, the prepared mixture (amino compound + polymer) was examined as an oil viscosity improver (added as 7.8% of oil; (SN 500) that was produced at Dorah refinery). 0.4 g of prepared DQC was mixed with 6.6 g of polymer P20, then the mixture was added to 82.72 g of basic oil (SN 500) at temperature 80 °C along with continuous mixing at reflux apparatus for three hours, then the mixture was cooled to room temperature.
Diagnosis and evaluation
Diagnosis using FT-IR: The tetra amino compound was diagnosed using IR spectrophotometer (NICOLET 380 thermo scientific) at dept. of environment and corrosion petroleum research and development center.
Analysis by C.H.N: The test was done for prepared product then the result was compared with the calculated theoretical portions.
Kinematic Viscosity Test: The test was done before and after adding the viscosity improver to oil at temperature 40 °C, 100 °C according to ASTM (D 445) at dept. of analytical labs at petroleum research and development center.
Flash Point: The test was done for oil before and after adding viscosity improver according to ASTM (D92) at dept. of analytical labs at petroleum research and development center.
Flow Point: This test was done for oil before and after adding viscosity improver according to ASTM (D97) at dept. of analytical labs at petroleum research and development center.
Viscosity Index: The test was done for oil before and after adding viscosity improver according to ASTM (D2270) at dept. of analytical labs at petroleum research and development center.
Sulfated Ash Test: The test was done for oil before and after adding viscosity improver according to IP 163/78 at dept. of analytical labs at petroleum research and development center.
Specific Gravity Test: The test was done for oil before and after adding viscosity improver according to ASTM D 4052, IP 190/79, and ASTM D1298-99 at dept. of analytical labs at petroleum research and development
Results and discussion
Preparation and evaluation of DQC
The quaternary ammonium compound is a surface-active agent that can be prepared depending on activity of nonbonding electrons on both two nitrogen atoms of EDTA by the reaction with 16th carbon atoms alkyl halide where the produced compound is evaluated by FT-IR spectrum. Figure 1 shows the FT-IR chart for DQC product compound which shows the positions of functional groups and Table 1 represents the details of functional groups positions.
Also, the DQC produced compound tested by CHNSO analyzer. Table 2 shows the CHNSO analyses for DQC.
Solubility of DQC
The solubility of prepared DQC was tested in Toluene, xylene and base lubrication oil where results indicates successful solubility of DQC in Toluene and xylene while DQC was not soluble in base lubrication oil.
Selection of polymer
Many experiments were carried out on four types of polymers in order to select the polymer compatible with DQC in solvent and base lubricant oil. These polymers included Poly Urethane, Epoxy Resin, Silicon Oil and Polyoxyethylene P20.
Table 3 shows the solubility tests where the results indicated that Poly Urethane, Epoxy Resin, Silicon Oil, Polyoxyethylene P20 successfully soluble in toluene, xylene and mystelene at room temperature but Poly Urethane, Epoxy Resin, Silicon Oil were not compatible with base lubricant oil. Polyoxyethylene P20 was thermodynamically stable in toluene, xylene and mystelene and base lubricant oil, it also made stable mixture with solvent and base lubricant oil.
Selection of viscosity improving mixture
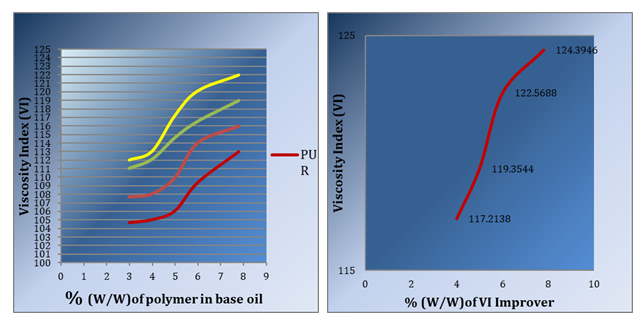
Figure 2 shows the relation between polymeric solution ratio [(Poly Urethane + Mystelene), (Epoxy Resin + Mystelene), (Silicon Oil +Mystelene), (Polyoxyethylene P20+ Mystelene)] and Viscosity Index (VI), Mystelene were chosen as solvent because its high boiling point making it safe at high temperatures. P20 Improved VIby 10 degrees while other polymers improved VI by (2–8) degrees.
Figure 3 shows the relation between VI improver weight percent (VI improver includes of P20 + Mystelene + DQC) and Viscosity Index where the results indicates that addition of 7.8% of improver to the base lubricant oil improves VI by 14 degrees.
Tables 4 and 5 show the effect of VI improver on general properties of base lubricant oil.
Conclusion
1. During practical experiments to prepare an improver to viscosity index, the DQC was prepared and used as (7.8 % w/w) which lies among the permitted concentrations to be added to lubrication oils.
2. The prepared viscosity index improver was used as a single additive and was not added as package.
3. During studying the addition of viscosity index improver to oil, the other oil specifications such as pour point, flash point etc. were not affected by this edition.
Recommendations
1. Considering DQC as an active material in increasing viscosity improver.
2. Considering the economic usefulness of the product as viscosity improver for oil comparing it with other viscosity improvers currently in use in Iraqi refineries taking in consideration the cost that the prepared product may spare concerning concentration as minimum and maximum addition.
3. Considering study results successful since the tests results were proven successful during practical tests, the study was presented to the “Central organization for standardization and quality control “to get a patent and considering the intellectual property of used chemicals.
Acknowledgements
I would like to express my acknowledgments to all staff in AL-Dura refinery.
How to cite this article: Zaydoon Kh. Kuraimd, Qays Mothanna Ammouri, Thabit A. Ahmed, Hadeel A. Husain Husain. Synthesis of viscosity index improver for motor lubricating oil. Eurasian Chemical Communications, 2021, 3(7), 477-483.
Copyright © 2021 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.