Document Type : Original Research Article
Authors
1 Chemistry Department, College of Sciences, Baghdad University, Baghdad, Iraq
2 Ashur University college, Emeritus professor at College of Women Sciences, Baghdad University, Baghdad, Iraq
Abstract
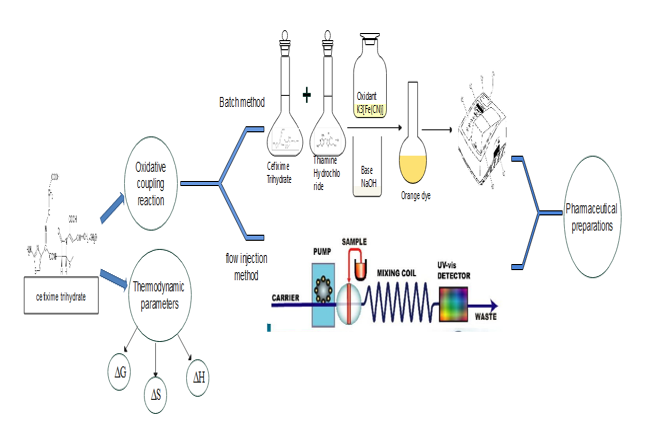
This study aimed at developing pectrophotometric methods for the determination of Cefixime Trihydrate [CFT] by simple, sensitive batch and flow-injection analysis in its purest form and tablet formulations. These developed methods were based on an oxidative coupling reaction of CFT with thaimine hydrochloride in the presence of a basic medium and using potassium ferricyanuide as an oxidant to produce a stable water-soluble, intense orange dye with a maximum absorption at 440 nm. Beer's law was followed over the concentration range [2.5-75 and 10-400 µg.mL-1] of CFT with limits of detection of 0.7223 and 2.6145 µg.mL-1 of CFT for batch and FIA methods, respectively. The average sample throughput for the FIA was 68 h-1. The effects of various chemical and physical experimental parameters on the development and stability of the colored product were carefully investigated. CFT estimated in commercial tablets forms was successfully applied using these methods, in which the results were in good agreement with those produced using the official procedure. Thermodynamic parameters, including the free energy changes [∆G], enthalpy [∆H] and entropy [∆S] were determined at different temperatures for the reaction product.
Graphical Abstract
Keywords
Main Subjects
Introduction
Cefixime trihydrate [CFT] is used in the treatment of uncomplicated Neisseria gonorrhoeae vaginal infections, upper respiratory tract bacterial infections, and simple Neisseria gonorrhoeae genital infections [1]. It has the chemical formula C16H15N5O7S2.3H2O (Figure 1) [2].
Determination of CFT concentration in pharmaceutical preparations was previously performed using high-performance liquid chromatography [HPLC] [3], UV spectrophotometric [4-6]. Also, many visible spectrophotometric methods have been presented in literature for determination of CFT including, chemilumences [7], Cloud point extraction [8,9], diazonium reaction [10], ion pair reaction [11] and complex formation [12]. Although the oxidative coupling reaction is a widely used method for the determination of many drugs [13,14], the studies reported a few oxidative coupling reactions [15] available for the determination of CFT; therefore, a simple reaction of oxidative coupling using Thiamine hydrochloride [TMH] is described in this study as a new chromogenic reagent with potassium ferricyanide and alkaline medium.
Thiamine HCl is also known as vitamin B1, as a drug compound with the chemical formula C12H17N4OS.HCl [1] (Figure 2).
A rapid oxidative coupling reaction of CFT with the TMH [as a green reagent] was used for sensitive assays of trace amounts of CFT in pure form and dosage. The formation orange-dye was determined spectrophotometrically using both batch and flow injection methods; thermodynamic parameters [free energy changes ΔG, enthalpy of formation ΔH and entropy ΔS] have also been studied at different temperatures.
Experimental
Apparatus
1- Digital single-beam spectrophotometer [Shimadzu UV-Visible 1240] was used to measure the absorption and the spectra measurements were performed by a shimadzu UV-1800 digital double beam spectrophotometer using a quartz cell [1 cm bath length] for batch method. In FIA method, all the absorbance measurements were carried out using Quartz flow matched cells with 10 mm path length and 50 µL internal volume.
2- A six-channel peristaltic pump [Gilson, minipuls 2, beltline modulation. W1. 53562, France] used for reagent pumping solutions. A 6-way valve for injection with various loops [Rheodyne, Model 7000 Stream Switching Valve-USA] was used for injected samples.
A three-channel manifold (Figure 3) was employed for the FIA spectrophotometric determination of CFT drug. For the peristaltic pump, flexible 0.5 mm [internal diameter] of vinyl tubing has been employed and a Teflon reaction coil [RC] with an internal diameter of 0.5 mm was used.
3- Thermostatic water bath [15 °C -90 °C] model; YCW-40 M, Gemmy industrial corp., Taiwan.
The solution of potassium ferricyanide reagent was transported through one of the three channels. The [TMH] reagent was transported through the second and the solution of sodium hydroxide was passed through the third channel and was combined with the mixed solutions of injected CFT, oxidant and the TMH reagent and the reaction then completed at the reaction coil. With a total flow rate of 3 mL/min, the resulting solutions have been driven into the detector by peristaltic pump. The colored product formed [orange dye] was measured at 440 nm. and at a temperature of 25 °C.
Materials and chemicals
All reagents used were of analytical grade and distilled water was used in this work. The state company for Drug Industries and Medical Appliance, [SDI, Samara-Iraq] supplied the work with pharmaceutical grade of CFT and excipients, commercial pharmacies provided the Pharmaceutical dosage forms containing CFT. For this study, two types of tablets [Winex, 400 mg, Tabuk pharmaceuticals Co., Saudi-Arabia and Cefix, 400 mg, Pharma International Co., Jordan], each contains CFT as its active constituent, were used for analysis.
Preparation of solutions for both batch and flow injection methods
The stock solution of Cefixime trihydrate [1000 µg.mL-1 = 1.9704 x 10-3 M]: A standard CFT [SDI] was weighted [0.10000 g] and dissolved in a certain volume of methanol [5 mL] then was completed to 100 mL in a volumetric flask with distilled water. Stock solutions of 500 µg.mL-1and 100 µg.mL-1, were prepared directly by dilution of 50 mL and 10 mL of 1000 µg.mL-1 of the stock solution in 100 mL volumetric flask with distilled water respectively.More dilute solutions can be prepared daily by appropriate dilution using distilled water.
Thiamine Hydrochloride reagent solution [1x10-2M]: After dissolving 0.3373 g of standard TMH [SDI] in distilled water, the volume was completed to 100 mL volumetric flask using the same solvent.
Potassium ferricyanide solution [1x10-2M]: It was prepared by dissolving 0.3293 g of pure K3[Fe[CN]6] [BDH] in distilled water then in a 100 mL volumetric flask, complete the volume to the mark with the same solvent.
Sodium hydroxide [BDH] stock solution [0.5 M]: Prepared by dissolving 2 g of NaOH in 100 mL volumetric flask and complete the volume to the mark with distilled water.
Pharmaceutical dosage formssolutions
For both winex and cefix tablets, ten tablets [400 mg CFT] were weighed and then finally powdered. The residue was washed and diluted to volume with distilled water to obtain 1000 µg.mL-1 of CFT. An amount of resultant powder equivalent to 0.1000 g of CFT was dissolved in 5 mL of methanol and the resultant solution was shacked and filtered into a 100 mL volumetric flask. For batch and FIA methods, more dilute solutions were prepared daily by appropriate dilution using distilled water.
Calibration graphs
A- General Procedure of batch method
A series of 20 mL standard flasks were filled with increasing volumes [0.1-3 mL] of 500 µg.mL-1 [9.852x10-4 M] CFT. 1 mL potassium ferricyanide oxidant 1x10-2 M, 1 mL TMH reagent 1x10-2 M, and 1 mL sodium hydroxide 0.5 M. The contents of the flasks were then diluted to the mark with distilled water, mixed thoroughly, and left at room temperature [25 °C] for 15 minutes. The absorbance of the orange dye formed was measured at 440 nm against a reagent blank containing all materials except CFT. For the optimization of conditions and all subsequent experiments, a 1 mL of [500 µg.mL-1 = 9.852x10-4 M] CFT in a final volume of 20 mL was used with a calibration graph and the regression equation calculated.
B- General procedure of FIA
Working solutions of CFT in the range of [10–400 µg.mL-1] were freshly prepared and injected in triplicate through the FI manifold to test the method's linearity. A 150 µl portion of CFT was injected into the stream of oxidant solution Potassium ferricyanide 1x10-2 M, which was then mixed with the reagent solution TMH 1x10-2 M, and the mixture was then combined with a stream of 0.5M sodium hydroxide [Figure 3]. The orange product's absorbance was measured at 440 nm, and a calibration graph is shown in Table 1. The conditions were optimized using CFT at a concentration of 100 µg.mL-1.
Results and discussion
A- batch spectrophotometric determination
The factors that influence the sensitivity and stability of the colored product produced by the reaction of the CFT and TMH in the presence of an oxidant and in an alkaline medium have been thoroughly investigated. A typical spectrum for the colored dye formed versus reagent blank (which has a minimum absorbance at λmax 440 nm) is showed in Figure 4.
The optimization of conditions for the proposed method was important to have a complete reaction formation, highest sensitivity and maximum absorbance. 0.01M of TMH [0.1- 4 mL], 0.01 M of K3[Fe[CN]6] [0.1- 4 mL], and 0.1 M of NaOH [0.1- 4 mL] were found to be the best experimental conditions for CFT determining by adding appropriate volumes of their solutions to a fixed concentration of CFT and measuring the absorbance at 440 nm. against a reagent blank for each one. The results (Figure 5) showed that for 25 µg mL-1 of CFT, 1.0 mL of 0.01M TMH, 1.5 mL of 0.01M K3[Fe[CN]6], and 1 mL 0.1M NaOH gave the highest color intensity and led to the highest color stability of the dye product.
After CFT [25 µg.mL-1] had been reacted immediately with oxidant and reagent in aqueous basic medium, the color intensity was increased gradually and reached maximum, then became stable after 15 min. For about 120 minutes, the absorbance remained stable. The effect of order of addition was also investigated using various orders of addition, with the results revealing that the order of reagent addition [Drug+ Oxidant+ TMH+ Base], gave a maximum absorbance and stability of the product. The effect of temperature on reaction development was investigated at three different temperatures [5, 25, and 55 °C]. In practice, the calibrated flasks produced higher absorbance at room temperature [25 °C] than at low temperature [5 °C] or high temperature [45 °C], so the room temperature was chosen for all subsequent experiments.
Using a 9.8522 x 10-4 M solution of equimolar for each CFT and TMH reagent, the product structure was determined using the mole continuous variation method [Job's method] and the molar ratio method [16]. The obtained results (Figures 6 and 7) showed that a 1:1 orange dye was formed between CFT and TMH reagent, which probably has the following structure (Scheme 1).
The FT-IR spectra of the dye product showed a new stretching single band at 3463 for NH group as shown in Table 1.
The resulting product was water soluble. The apparent stability constant was calculated by comparing the absorbance of a solution containing stoichiometric amount of CFT [9.8522x10-4 M] and TMH [9.8522x10-4 M] in a final volume of 20 mL [As] with that of solution containing a five-fold excess of TMH reagent in a final volume of 20 mL [Am] and in accordance with the analytical procedure the average of stability constant [K] for three different concentrations 20, 40 and 90 µg.mL-1 = 2.6490x104 L.mol-1 , where [K=[1-α]/ α2C] and α=Am-As/Am [17]. The high value of stability constant indicates that the reaction product is stable.
The calibration graph intercept, slope, and correlation coefficient values were calculated and listed in Table 3, and the results were compared to those obtained using the FIA proposed method. A recovery test for CFT was performed in the presence of [10-fold] of excipients in order to examine the methods usefulness and freedom from interference by tablet additives. The high percentage recoveries indicated that there was no interference and that the method had good selectivity for analyzing CFT in its various dosage forms.
FIA-spectrophotometric determination
The development of the normal flow injection [nFIA] procedure was based on the previous batch method for CFT assay. The choice of the best manifold for a flow system is very essential, so various manifold reactions were used to carry out various paths, and the results revealed that the manifold in Figure 1 was chosen for further experiments because it gave the best absorbance of the reaction's product.
Maximum absorbance intensity was accomplished when a 150 µL of (100 µg.mL-1) CFT was injected into the stream of 0.01 M of oxidant which mixed with the reagent solution 0.01 M the mixture then mixed with (0.1 M) of NaOH solution. As shown in Figure 1, the reaction was completed in reaction coil; each sample was injected three times, and different physical or chemical parameters optimized by changing one while leaving the others unchanged.
Chemical parameters optimization
The chemicals variables effect
To improve the sensitivity of the suggested reaction, chemical parameters were optimized and studied. The optimal concentration of the reagent [TMH] was determined by passing multiple concentrations in the manifold ranging from 0.0005 to 0.05 M. The results in Figure 7 showed that a concentration of 0.005 M produced a high absorbance and good accuracy; therefore, it was chosen for future testing. Different concentrations of oxidant [potassium ferricyanide] were also examined, with concentrations ranging from 0.0005 to 0.05; the optimum concentration of 0.005 gave the best results and minimum blank value as shown in Figure 7 and was considered as optimum value. On the other hand, the effect of sodium hydroxide concentration which was important factor on reaction development was also investigated in the range of concentrations [ 0.01 M- 1 M] and the optimum concentration [ 0.2 M] seems to be best as shown in Figure 8.
The manifold parameters optimization
Physical factors such as flow rate, injection sample volume, and reaction coil length were investigated under optimal reagent concentrations. In the range of 0.5-4 mL.min-1, the influence of total flow rate on the sensitivity of the colored reaction product was examined. The results revealed that a total flow rate of 3 mL.min-1 produced the maximum absorbance (Figure 9) and was employed in all following experiments.
The injection sample volume was changed between 50 and 250 µl by varying the length of the sample loop. The results (Figure 10) revealed that the 150 µl injected sample had the highest absorbance.
The length of reaction coil was an important parameter affecting the sensitivity of the colored reaction product and was studied in the range of 25-250 cm. The results showed that a coil length of 100 cm produced the maximum absorbance then the absorbance decreased because of the increase in dispersion, as shown in Figure11, same length was employed in all following experiments.
The sampling frequency was investigated under the optimum conditions by monitoring the time from sample injection to maximum absorbance [53 seconds], resulting in a sample throughput of 68 samples per hour.
Using the optimum reaction conditions and the FIA manifold in Figure. 1, the dispersion of the colored product was determined to be 1.475, indicating a minimal dispersion in FIA that allows for high sample rates and sensitivity [16].
Characteristics of analysis
Under optimal conditions, each method's analytical features such as linear range, detection range, correlation coefficient, and relative standard deviation [RSD] were calculated [Table 3]. When comparing the batch and FIA procedures, the latter is more convenient due to its speed and the greater linear range of the calibration graph was obtained.
Accuracy and precision
The accuracy and precision of the proposed methods for determining CFT using both batch and normal flow injection methods were investigated under optimum conditions using three different concentrations of standard CFT in both methods. Table 4 shows the E.%, Rec.%, and RSD.% of three CFT determinations suggesting good recoveries and low RSD% values for that the developed methods was accurate to determine the micro amount of CFT in pure and pharmaceutical preparations.
Applications of analysis
By analyzing three different concentrations of tablets utilizing the analytical processes directly, batch and FIA techniques for determining CFT in pharmaceutical dosage forms were effectively used [Table 5]. The results were compared with those obtained using the British pharmacopeia standard method [19], to evaluate the efficacy and success of the recommended approaches. Calculating the Student's t- and F-values [20] was used to evaluate the current method's performance. Table 6 shows that the estimated t- and F-tests did not exceed the theoretical values and there is no significant difference between either methods in accuracy and precision in determination of CFT in pharmaceutical formula.
Thermodynamic studies
Thermodynamic studies [the free energy changes ΔG, enthalpy of formation ΔH and entropy ΔS] have been considered at different temperatures [15 °C, 25 °C, 35°C and 45 °C], The Van't Hoff equation [21] illustrates the relationship between temperature and the stability constant [K]. A plot between lnK versus 1/T gave a straight line, a slop was equal to -∆H/R [where ∆H= enthalpy changes and R is the gas constant 8.314 J.mol-1.k-1], The intercept was equal to the standard entropy changes divided by the gas constant ΔS/R [Figure 12]:
The reaction's Gibbs free energy [G] was also estimated using the Gibbs-Helmholtz equation [22]: G= H-TS, and all thermodynamic parameters are presented in Table 7.
The negative values of ΔG were indicated that the oxidative coupling reaction is spontaneous, while the negative value of ΔS means decreasing in disorder of the reaction because of the thermal decomposition of complex as the temperature rises and suggest a fast reaction that may be studied using the spectrophotometric flow injection method. The negative value of ∆H, on the other hand, denotes that the reaction is exothermic and that heat is released as a result of the reaction, and this may explain the reason for decreasing on the stability constant with increasing in temperature.
Conclusion
For the determination of Cefixime trihydrate in pure and pharmaceutical preparations, batch and FIA spectrophotometric methods were proposed. These methods have many advantages of simplicity, speed, accuracy and the use of inexpensive equipment. The speed of the proposed methods and its precision make them suitable for the quality control of formulations containing this drug. The wide linear range of the proposed methods that followed Beer's law gave a good application for the pharmaceutical preparation, and can be used as a reliable and advantageous alternative to the other previously exported methods for routine analysis of CFT in pharmaceutical samples using the non-toxic reagent which is a drug [thiamine hydrochloride] and this was an important factor for improving the green chemistry. Also, the calculations of thermodynamic studies at different temperatures obtained indicated that the reaction was spontaneous and fast and did not need a high temperature to proceed the reaction.
Acknowledgements
The authors are grateful for the facilities by Department of Chemistry, College of Sciences, University of Baghdad and for the state company for Drug Industries and Medical Appliance, [SDI, Samarra-Iraq] for supplying the work with pharmaceutical standard materials.
Orcid:
Hind Sadiq Al-ward: https://orcid.org/0000-0003-2500-1504
How to cite this article: Hind Sadiq Al-ward*, Mohammed Rifaat Ahmed, Mouayed Qassim Al-Abachi. Thermodynamic study and spectrophotometric determination of cefixime trihydrate in pure form and pharmaceutical tablets using batch and normal flow injection analysis. Eurasian Chemical Communications, 2021, 3(7), 495-507. Link: http://www.echemcom.com/article_132688.html
Copyright © 2021 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.