Document Type : Original Research Article
Authors
1 Department of Chemistry and Biochemistry, College of Science for Women, Baghdad University , Baghdad, Iraq
2 Department of Chemistry, College of Science for Women, University of Baghdad, Baghdad, Iraq
Abstract
Both thyroid diseases (TD) and diabetes mellitus are common endocrine disorders that often appear in clinical practice (DM). It is more common for persons with type 2 diabetes to have hypothyroidism or hyperthyroidism (T2DM). A considerable influence on blood glucose, lipid, and protein metabolism, as well as many other organs and tissues, is exerted by thyroid hormones in the blood (T2DM). It has been shown that in people with hyperthyroidism, the presence of osteopontin increases, and in those with hypothyroidism, it decreases, indicating that it may be a novel way to find thyroid illness. A total of one hundred twenty (120) subjects were included in current study. Variables like (gender, age, BMI, thyroid hormones, FBS, HbA1c, lipid profile and OPN) were recorded and documented from participant included in this study. The results of current study displayed there was a significant differences between osteopontin and body mass index in patients suffering from thyroid dysfunction with diabetes (-0.37) with (P≤0.05), T3 was (0.32) and FBS was (0.35) both of them recorded significant differences and (P≤0.05) in patients with diabetes type II. TSH, T3 and T4 findings were high significant differences with OPN in patients with thyroid dysfunction with diabetes with (-0.89), (0.9) and (0.89) respectively. While other parameters recorded no-significant differences.
Graphical Abstract
Keywords
Main Subjects
Introduction
There are two of the most common endocrine diseases encountered in clinical practice that are related to thyroid disease (TD) and diabetes mellitus (DM) [1]. Thyroid disease and diabetes mellitus are inextricably connected. According to research [4], thyroid problems are more common in people with diabetes mellitus, and vice versa.
T2DM (a.k.a. type 2 diabetes) increases the risk of hypothyroidism and hyperthyroidism. The thyroid hormones in the blood have a large impact on many different organs and tissues, including glucose, lipid, and protein metabolism. This may result in more carbohydrate consumption and a worsened glycemic control in diabetics (T2DM) [11]. Thyroid function can affect carbohydrate metabolism through the effect of insulin, which can lead to the progress of T2DM if thyroid and insulin function are both out of whack (T2D). Carbohydrate metabolism is dependent on thyroid hormones [8]. Hyperthyroidism (excess thyroid hormone) can cause hyperglycemia by impairing insulin production, action, and clearance, as well as many other aspects of glucose metabolism. On the other hand, hypothyroidism (a lack of thyroid hormone) can impair insulin action and metabolism, leading to insulin resistance [13]. Many diabetics acquire thyroid dysfunction symptoms over time. Insulin resistance is a major contributor to hypothyroidism in the people with type 2 diabetes. In diabetic individuals, hypothyroidism exacerbates dyslipidemia, hypertension, and cardiovascular disease [5]. Diagnosing and treating hypothyroidism in diabetic individuals is critical to minimize the development of diabetes complications. A simple blood test for hypothyroidism is readily accessible and can be used to diagnose the condition [7]. Diabetes is one of the fastest-growing non-communicable metabolic syndromes, characterized by elevated blood glucose levels caused mostly by insulin secretion or action suppression [14]. Obesity is described as a state of being overweight or obese, and type 2 diabetes is likely to be the largest epidemic in human history [19].
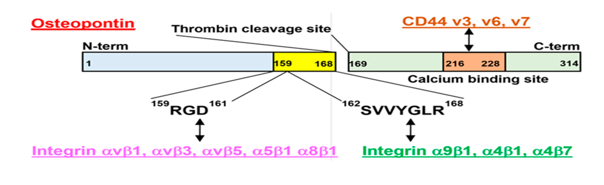
OPN is a glycosylated extracellular matrix glycoprotein produced by osteoclasts, osteoblasts, immunological cells, epithelial cells, endothelial cells, and extraosseous cells (kidneys, skin as well as lungs) [9]. Osteopontin has been reported to be up regulated in some people with hyperthyroidism and down regulated in hypothyroid patients, suggesting that it could be a novel marker in the identification of thyroid illnesses [3]. Osteopontin is intricate in a variety of physiological as well as pathological processes and disorders, including chronic inflammations such as Crohn's disease, obesity, Graves' disease, various autoimmune diseases, cancer, cardiac fibrosis, and atherosclerosis (29). Osteopontin is a glycophosphoprotein that is highly phosphorylated and has high aspartic acid content. The human OPN protein includes both O-linked and N-linked oligosaccharides and is 314 amino acids long. Figure 1 shows the structural shape of OPN.
Osteopontin is a multifunctional protein that is important for biomineralization, bone remodeling, as well as periodontal remodeling when a mechanical strain and stress (orthodontic movement of teeth) are present [16]. OPN also controls both the adaptive immune system as well as innate immunity. OPN stimulates a cell-mediated immune response in a parallel way similar to T cell helper 1 cytokines [10].
In epithelial tissues, Osteopontin inhibits calcium crystal formation and aggregation [18]. OPN has also been related to a variety of illnesses, where it controls and propagates inflammatory responses in T-cells, macrophages, as well as dendritic cells via regulating a variety of cellular adhesion, migration, and survival processes. OPN's pleiotropy may be explained by the post-translational modifications, many isoforms, as well as cell types with which it interacts. Chronic inflammatory illnesses including Crohn's disease [2], cancer [6], aortic abdominal aneurysms, atherosclerosis, as well as autoimmune diseases like lupus, have been related to OPN plasma levels [12,15].
In conclusion, OPN is a protein with a wide variety of activities, including bone mineralization control, cell adhesion and migration enhancement, and recruitment of macrophages [17].
Subjects, materials and methods
A case-control study was done at the Specialized Center for Endocrinology and Diabetes – Al-Kindi Specialized Hospital in Baghdad. The samples were collected from the first of January 2021 to first of April 2021. Variables like gender, age, BMI, thyroid hormones, FBS, HbA1c, lipid profile, OPN and ADP were recorded and documented from participants in this study.
A total of 120 subjects participated in the current study. Thirty patients were suffering from diabetic mellitus type 2, thirty were apparently healthy subjects with matched gender and age, thirty patients had thyroid dysfunction another thirty subjects included of patients had thyroid dysfunctions and diabetes mellitus type 2.
A kit was used to the quantitative detection of human osteopontin (OPN) concentrations in human samples (Cat No.CSB-E08392h–USA). Cobas e 411 analyzer was applied for TSH, T3, T4 in vitro thyroxin immunoassay for quantitative evaluation of human serum and plasma. The Cobas e immunoassay equipment is compatible with the electrochemiluminescence immunoassay "ECLIA." (Roche/Germany). Cobase c 111 system was used for vitro glucose determination in human serum, plasma, FBS and HbA1c (Roche/Germany). Cobase c 111 Roche/Hitachi cobas c system was utilized, which is an in vitro test for the quantitative detection of lipid profiles (Roche/Germany).
Results and discussion
The results of the current study are summarized in Table 1, which show that there was a significant difference between OPN and BMI in patients suffering from thyroid dysfunction with diabetes (-0.37) with (P≤0.05); T3 was (0.32) and FBS was (0.35), both of which showed significant differences and (P≤0.05) in patients with diabetes type II, while TSH, T3 and T4 findings were high significant differences with OPN in patients with thyroid dysfunction with diabetes with (-0.89), (0.9) and (0.89), respectively. Other parameters displayed no-significant differences.
The current findings, which are based on analysis of the results of study groups are demonstrated in Table 1, showing there was a significant differences between OPN and BMI in patients group of thyroid dysfunction with diabetes, this result is similar to other studies [20-22], reporting that Adipose tissue metabolism has gained attention due to increased interest in understanding the etiology of obesity, as well as for therapeutic implications and potential medication discoveries. The levels of thyroid-stimulating hormone (TSH) are higher in severely obese people, which is linked to a little higher level of T3 (triiodothyronine). To find out whether there is a connection between components of the metabolic syndrome and thyroid function, researchers compared thyroid function in obese and/or diabetic individuals with that of healthy normal weight counterparts. Ischemic (coronary) disease is the single most important risk factor for premature death in the elderly [23], which is related with insulin resistance and obesity, and according to another study [24], obesity is substantially associated with an elevated risk of hypothyroidism.
TSH, T3 and T4 findings were high significant differences with OPN in patients with thyroid dysfunction with diabetes with (-0.89), (0.9) and (0.89) respectively. This finding supports those of [25,26], who found that it is caused by excessive thyroid hormone levels, excessive bone loss and a high level of biochemical indicators of bone turnover occur. OPN is one of the tests used to determine bone turnover. There is a positive correlation between OPN concentrations and bone mineral density, and a negative correlation with bone turnover. Osteopontin has a favorable relationship with the thyroid hormones T3 and T4, and its pro-inflammatory impact has been linked with thyroid illness [27]. The fundamental pathophysiology of the relationship between osteopontin and thyroid dysfunction is yet unknown. A lot more research is required to see if osteopontin concentration is affected in all cases of the thyroid malfunction (particularly in subclinical cases) [27].
Our results did not agree with those of [28] who showed no significant connection between OPN and (FBS and HbA1c).
Conclusion
The two most often diagnosed endocrine diseases in clinical practice are diabetes mellitus and thyroid ailment. There is speculation that hyperthyroidism is a new thyroid disease indicator, as individuals with the condition have been found to have higher levels of osteopontin. However, those with hypothyroidism have been reported to have lower levels of osteopontin, fueling the theory that this is a previously unknown thyroid disease indicator.
Acknowledgements
First and foremost, I do praise God (Allah), without his love, grace and mercy, none of my achievements would be possible. My gratitude is to my role model prophet "Mohammad" (peace be upon him, his relatives and companions) who said: (seek knowledge from the cradle to the grave). I would like to acknowledge with deep appreciation and gratitude the invaluable help of my supervisors (Prof. Dr. Sanad Baqir Mohammad) whose hands were always ready for help. My appreciation goes to the staff of the Department of Chemistry in the College of Science for Women for all the help they provided during my study. I am indebted to the staff of The Specialized Center for Endocrinology and Diabetes Al-Kindi Teaching Hospital who have been helpful all the time. Finally, I would like to thank all patients and their families who helped me by giving me samples and information that helped me in my study.
Orcid:
Sabah Qusay Abd-Alhussain:
https://orcid.org/0000-0001-7407-238X
Sanad Baqir Mohammed:
https://orcid.org/0000-0001-8739-9939
----------------------------------------------------------------------------------
How to cite this article: Sabah Qusay Abd-Alhussain*, Sanad Baqir Mohammed. Association of osteopontin with thyroid dysfunction in type П diabetes mellitus. Eurasian Chemical Communications, 2021, 3(11), 806-811. Link: http://www.echemcom.com/article_138175.html
----------------------------------------------------------------------------------
Copyright © 2021 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
