Document Type : Original Research Article
Authors
Department of Chemistry, College of Science, University of Baghdad, Baghdad, Iraq
Abstract
By diazotization and coupling for 7-Bromo-8-hydroxyquinoline and sulfamethoxazole, the novel [4-(5-(7-Bromo-8-hydroxyquinoline) azo) sulfamethoxazole] (HAS) has been produced. To make new complexes, the ligand (HAS) was reacted with [Pd (II), Pt (II), Ni (II), and Co(II)]. Spectroscopic methods, thermal analysis, molar conductance data, and magnetic measurements were utilized to analyze both the (HAS) ligand and metal complexes of the ligand. The technique of mole ratio was used to determine the stoichiometric of the complexes, which was [1:2] [M:L] for the complexes of Pt(II) and Pd(II) exhibited square planar geometry, whereas Ni(II) was determined to be octahedral and Co(II) was determined to be distorted octahedral geometry. For HAS ligand and its complexes, the dyeing performance, anti-bacterialand and anti-fungal properties were studied. The Platinum complex was also investigated as an antioxidant and anti-cancer agent, whereas cobalt complex was investigated as an anti-inflammatory agent. The ligand was put to the test as an indicator.
Graphical Abstract
Keywords
Main Subjects
Introduction
A crucial role has been played by azo dyes that contain heterocyclic rings in developing coordination chemistry [1]. Azole chemicals, on the other hand, have been combined with different biological processes, involving RNA, DNA, nitrogen fixation, and cancer [2]. The antibiotic sulfamethoxazole (SMX) is a bacteriostatic agent that is commonly utilized to treat a variety of infections. Its widespread usage in even animals and humans produces unmetabolized and active metabolites with a wide range of biological effects [3]. Sulfamethoxazole (SMX) is a commonly prescribed and utilized antibiotic in both veterinary medicine and human, as well as a common sulfonamide in both aquatic and terrestrial settings. In a European review of pharmaceuticals and personal care products, it was also named to a top ten list of high-priority medications [4]. 8-Hydroxyquinoline compounds have been found to have several antibacterial, antioxidant, anticancer, antiinflammatory, and antineurodegenerative properties. When compared to the original chemical, the halogenated 8HQ, particularly 7-Bromo-8HQ, had a strong antigrowth effect against Gram-negative bacteria [5]. The main goal of our research is to synthesize new complexes of [Ni (II), Co (II), Pt (II), and Pd (II)] with the ligand [4-(5-(7-Bromo-8-hydroxyquinoline) azo) sulfamethoxazole] (HAS). The ligand and its complexes were identified by different physicochemical methods. The antioxidant, anticancer, antibacterial, antifungal, and anti-inflammatory properties were investigated. In addition, the dying performance and the ability for the ligand to act as an indicator were studied.
Experimental
Instruments and materials
The materials and solvents were used of the highest possible grade. Metal content and elemental analysis for the (HAS) ligand and complexes of the ligand were obtained using (C.H.N.S), and the proportion of metal in complexes was calculated using (Eure EA 3000 Elemental analyzer). FT-IR spectrophotometry was performed using a "Nova 350spectrophotomerer "Flam Atomic Absorption Spectrophotometer. UV-Visible Spectra for all the investigated compounds were recorded on the (SHIMADZU 1800- UV spectrophotometer) using ethanol in the range of (200-1100) nm using the SHIMADZU 8400s spectrophotometer in the region of (250- 4000) cm-1 with CsI. The 1HNMR spectra were obtained with tetramethylsilane as the internal standard on a BRUKER AV 400 Advance -III (400 MHz and 100 MHz) equipment. The metal content of the produced ligands and complexes was evaluated by thermal analysis (TG) (SDT Q600 V20.9 Build). Stuart melting point equipment was used to determine the melting points of all the compounds. In ethanol (10-3 M), the molar conductance of metal ion complexes was investigated using HI 9811-5" HANNA equipment. The Mohr technique was utilized to determine the chloride content of the materials examined. M.S.B. is the model's name. At room temperature, the magnetic susceptibility of the investigated complexes was evaluated utilizing the Auto Apparatus.
Synthesis of (HAS) ligand (Scheme 1)
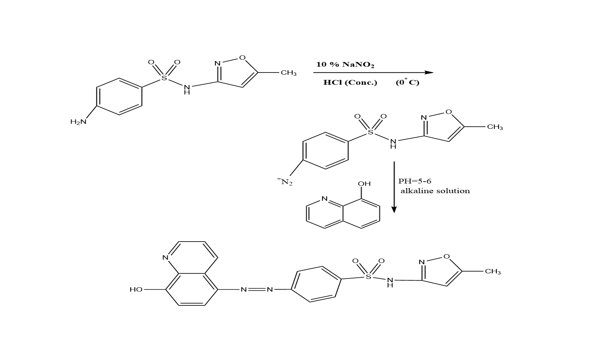
The ligand [4-(5-(7-Bromo-8-hydroxyquinoline)azo) sulfamethoxazole] (HAS) was synthesized as described in the literature [6] with minor modifications, as indicated in Scheme 1. The diazonium salt was synthesized by dissolving (2.53 gm; 0.01 mole) sulfamethoxazole in 10 mL deionized water and then adding 10 mL concentrated HCl, then cooled 10% NaNO2 solution was added to the mixture which had been chilled in an ice bath (0oC), with stirring for 1 hour. The solution of diazonium salt was slowly added to an alcoholic alkaline solution of 7-Bromo-8-hydroxyquinoline (2.2 gm; 0.01 mol). After that, the solution's pH was adjusted to (5-6). Leaved it overnight to precipitate, the colored powder that resulted was filtered and dried after washing it by ethanol: deionized water [1:1] and dry.
Metal complex synthesis
The ligand(HAS) in an ethanolic solution [0.9786 gm,0.002 M] is added to each of the ethanolic solution of the chloride salt for selected metals [Ni (II), Co (II) , Pt(II) and Pd (II)] (0.00l M) (0.237, 0.237,0.410 and 0.177,) gm respectively in terms of mole ratio [1:2] [M:L]. The combination then was refluxed for three hours, and the results were analyzed using a TLC technique. The color precipitate is filtered and rinsed with 100% ethanol many times. It was then allowed to dry. Table 1 appeared properties of the synthesized compound.
Anti-oxidant and radical scavenging activity
To test the reductive ability, 1 mL of each concentration of [Pt(HAS)2].5H2O (0.08, 0.16, 0.32, and 0.64 mg/mL). After mixing with 1 mL of 0.2M phosphate buffer (pH 6.6) and 1.5 mL of 1 percent potassium ferricyanide, the mixture was incubated at 50 °C for 20 minutes, as reported in the literature [7]. 1 mL of 10% trichloroacetic acid was added to the liquid to stop the reaction. 2.5 mL of the supernatant, 2 mL distilled water, and 0.5 mL of freshly made, 1 percent ferric chloride, were mixed after centrifugation for 10 minutes at 3000 pm. After that, at 700nm, the absorbance was measured. The Trolox solutions were treated in the same way (standards). All of the tests were carried out in duplicate.
Based on the stable DPPH free radical's scavenging activity, the antioxidant activity of the [Pt(HAS)2].5H2O and standard (vitamin C) were evaluated using the technique of [8]. In a test tube, 3.9 mL of DPPH solution was mixed with an aliquot of 0.1 mL of each of the two complexes or standard (0.625, 0.125, 0.250, and 0.500 mg/mL). A spectrophotometer was used to determine the absorbance of each solution at 517 nm after 30 minutes of incubation at 37 °C. Triplicates of all measurements were taken. The following equation was used to calculate the ability to scavenge DPPH radicals:
Anticancer effectiveness
The cytotoxic activity and mode of action of synthesized [Pt(HAS)2].5H2O complex were studied in this part against liver cancer cell line (HepG-2 ) and normal cell line (WRL68). MTT assays were used to evaluate [Pt(HAS)2].5H2O complex after incubation for 24 at 37 °C with concentrations of (6.25, 12.50, 25, 50, 100, 200 and 400) 𝜇𝑔/ mL. The chosen compound was discovered to have an inhibitory effect on the (HepG-2) and WRL68 cell lines. The extent of the toxic impact was calculated by comparing the percentage of inhibition to the control.
Antibacterial and antifungal of synthesized ligand and its complexes
The efficacy of antibacterial of the new azo ligand (HAS) and complexes of ligand was tested in vitro with two pathogenic microorganisms using a disk diffusion technique in [9] (10-3 M) ethanol as a solvent. Inhibitory zones (in mm) were measured after the Petri dishes were incubated for 24 hours at 37 °C (for bacteria) and 72 hours (for fungi), with sulfamethoxazole as a control for bacteria and fluconazole for fungi. The pathogenic microorganisms utilized were chosen because they may cause a wide spectrum of life-threatening disorders in living systems. There were gram-positive bacteria Staphylococcus aureus (Staph) and gram-negative bacteria Escherichia Coli (E.coli) while there was the one type of fungi (Rhizopus microsporus).
Burns healing (anti-inflammatory)
Burns's healing impact was tested to see if [Co(HAS)2(H2O)2].4H2O has anti-inflammatory activity. The ability of [Co(HAS)2(H2O)2].4H2O at (1.5 mM), silver sulfadiazine (positive control), and negative control to heal burns was investigated by determining the number of days required to recover the results.
Dying method
In the wool fabric, the coloring characteristics of the ligand (HAS) and its complexes were used. (0.3 gm) of azo dyes (HAS) ligand and its complexes were dissolved in an ethanolic solution of 10% NaOH, then added 50 mL of distilled water. After that, A piece of clean white wool fabric(10×10) cm, (30 gm ± 3 gm) that soaked in distilled water for (30) minutes was transferred to the azo dye solution and heated to 60 °C for 10 minutes. The soaked cotton fabric piece was washed three times with (250) mL distilled water containing 1 gm of soap to remove any non-reacting species. Finally, dyed raw was dried by hot steam [10].
Acid - base indicator
The ligand (HAS) was employed as an indicator in acid-base titration using (0.1M) HC1 vs (0.1M) NaOH and (0.1M) CH3COOH versus (0.1M) NaOH. When going from an acidic to a basic solution, the ligand has a distinct color in one solution and a dramatic color change in the other.
Results and discussion
In general, the complexes were created by reacting the desired metal salts with the azo ligand (HAS) at a mole ratio of [1:2] M:L, whereas the ligand was created by diazotizing sulfamethoxazole in acidic circumstances, and then it is paired with the Alkaloid 7-Bromo 8-hydroxyquinoline as a nucleophile. The data was obtained by atomic absorption to identify a percentage of the metal, and elemental analysis was under the usual formulae for the ligand (HAS) and its complexes of metals. All complexes have non-ionic properties (Table 1). In addition, the planned structure is backed up by spectroscopic measurement (FTIR, 1HNMR, and UV- Vis) and thermo-gravimetric analysis (TGA).
The mole ratio
The mole ratio approach was used to evaluate the stoichiometric reaction of the ligand (HAS) and its [Pd (II), Pt (II), Ni (II), and Co(II)] complexes. This is the most well-known approach for determining the type of complexes generated in the solution that requires separation when the amount of ligand is altered (0.25 mL) and the amount of metal ion is kept constant; this technique was utilized to test the absorbance vs molar ratio of the (M:L).[10]
The relationship between the (M:L) ratio and the absorbance is shown in Figure 1, while the results are collected in a Table 2. All synthesized complexes have a (1:2) (M:L) ratio.
Gibbs free energy and stability constant
The stability constant can be calculated spectrophotometrically [11]. For complexes with a mole ratio of [M: L] [1:2] was calculated using the equations below.
C= (molar concentration) for the complexes in molar (c = 10−3M)
α = degree of disintegration
Am = the solution's absorption which contains (volume of the metal and additional ligand)
As = solution absorption that consists (1:1) stoichiometric (M:L)
From Table (3) the stability increases in the following order : [Ni(HAS)2(H2O)2].4H2O > [Co(HAS)2(H2O)2].4H2O > [Pt(HAS)2].5H2O > [Pd(HAS)2].H2O
This equation yielded the thermodynamic characteristics of ΔG (Gibbs free energy). [12]:
ΔG = - RT ln K
Where:
R= constant of gas which equal 8.31 J. mole-1. K-1
(T) = Temperature absolute (Kelvin)
At the information we got through ΔG, the synthesis of all complexes was spontaneous. Table 3 shows the behavior of the thermodynamic parameters of ΔG:
The FT-IR spectra
The synthesized ligand (HAS) may subsist in two tautomeric variants that are conceivable (azo-hydrazone) as was shown in Scheme 2 and the deprotonation of the hydroxyl moiety in the 7-Bromo 8-HQ for two tautomers leads to anion [13].
In the spectra of FTIR for the ligand HAS, there was broadband at the range (3380-3244)cm-1 for hydroxyl (C8-OH) in quinolone due to forming a very estable hydrogen bond dimer as was depicted in the Scheme 3.The ligand (HAS) institute of five-membered chelate with hydrogen can be assorted into two kinds [13].
- An inter-molecular hydrogen bonding [O….H-O] resulted from the [C8-OH] moiety and (C8-OH) or nitrogen in (C=N)py for another 7-Bromo 8-HQ.
- An intra-molecular hydrogen bonding [O-H….N] has appeared between [C8-OH] and nitrogen in (C=N)py in the same 7-Bromo 8-HQ.
In the complexes spectra, this broadband was disappeared and a new band at (1247-1265)cm-1 was observed corresponding to (C-O), which indicates the deprotonation of [C8-OH] for 7-Bromo 8-HQ and acts as monobasic N,O-bidentate ligand [1]. As for (C=N)py the change it was not clear due to interfered with (C=N)iso.
No significant change was noticed in the bands (C-N=N-C), (N=N), (SO2), and (NH) when comparing the ligand spectrum (HAS) with the complexes spectra indicating not any part of coordination [14,15,1,16].
- The vibrations of ʋ (M-N)7-bromo8HQ and ʋ(M-O) may be attributed to certain new bands in the ranges of (414-432) and (570-590) cm-1 and cm-1, respectively [1]. Figures 2-6 show the results; FTIR spectra data of the bands of ligand (HAS) and their complexes were found in Table 4.
1H-NMR
The results obtained from the FTIR spectra are further supported by the 1H-NMR analyses. It is accomplished by comparing the changes in the 1HNMR spectra of generated complexes to those of free ligands. Table 5 shows the data for the shifting (𝛿) in ppm for several kinds of protons in the ligand (HAS) and its complexes for Pt(II) and Pd(II), while the 1HNMR spectra were obtained in DMSO-d6 solution (Figures 7-9).
The free ligand (HAS) shows the signal at (11.65), (2.52) ppm, and the varied signals observed in the range (7.11-8.06) ppm for NHsulfa, CH3oxazole, and Ar-Hbenzene respectively [17]. The free ligand (HAS) displays signals at (9.33)ppm and multiplet signals in the range (8.09-9.03)ppm for OH-quinoline and H-quinoline respectively [18]. All signals of the spectra of complexes of Pd (II) and Pt (II) were a light shift that refers to non-sharing of these groups in metal ions coordination while OH-quinoline signal in the ligand(HAS) is disappeared in the spectra of its complexes indicate sharing oxygen atom with metal ions in the coordination after losing proton [19].
Electronic-spectrum and magnetic properties
The geometry of solid complexes can be determined by looking at their electronic spectra. As a result, electronic spectrum features were concentrated on the discrepancies between the molecule's ground and excited states [20]. Table 6 lists the results of the electronic absorption for the ligand (HAS) and complexes of the ligand within (10-4) molar in ethanol at room temperature, whereas Figures 10-14 explain their spectra. The synthesized complexes' electronic spectra revealed novel bands. The positions and intensities of these bands are primarily determined by depending on the ligand field effect, metal-ion electron configuration, and complex stereochemistry.
The UV-Vis spectrum of the ligand (HAS) in ethanol within the range(200-1100) nm, Figure 10 displays mainly two peaks. The first peak for (HAS) ligand is at (244 nm, 40983 cm-1). This peak was pointed to the mild energy (π → π*) transition of benzene, naphthalene rings. The (n → π*) transition of intramolecular charge transfer via the azo moiety was revealed by the second peak at (405 nm, 24691 cm-1) [21].
In the electronic spectra of para magnetic(2.74)B.M [Ni(HAS)2(H2O)2].4H2O complex, the (λmax) peak belongs to the ligand (HAS) was shifted as anticipated to higher wave length by (61) nm after coordination. Three d-d transitions were expected for paramagnetic high spin (d8) octahedral geometry [22].
υ1=3A2g (F) → 3T2g (F)
υ2=3A2g (F) → 3T1g (F)
υ3=3A2g (F) → 3T1g (P)
Figure 11 showed only two weak transitions υ1 at (893 nm; 11198 cm-1) and υ2 at (762nm; 13123 cm-1) for the [Ni(HAS)2(H2O)2].4H2O while the υ3 transition peak may be cryptic by the (LMCT) peak at (466nm; 21459 cm-1)
The complex [Co(HAS)2(H2O)2].4H2O Figure 12 appears two main transitions:
υ1 =4T1g(F) → 4T2g(F) at (982 nm; 10183 cm-1)
υ2=4T1g(F) → 4A1g(F) at (862 nm;11600 cm-1)
and also the third transition peak at (496nm; 20161 cm-1) was obscured by the complex peak[LMCT]. The complex had a distorted octahedral geometry with paramagnetic properties [23].
In the spectra of the diamagnetic low spin (d8) Pd (II) complex (Figure 13), for square planar surfaces, the three (d-d) transitions were highlighted 1A1g→1A2g, 1A1g→1B1g and 1A1g→1E1g [10].
Only two transitions were observed at (971 nm; 10298 cm-1 and 839 nm; 11918 cm-1) for [Pd(HAS)2].H2O which belongs to 1A1g→1A2g and 1A1g→1E1g while the transition 1A1g→1B1g is too weak to appear. But regarding the peak at (460 nm; 21739 cm-1) was attributed to LMCT.
The value of the magnetic measurement for low spin d8 Pt(II)- complexes is zero, which is approaching the value of the four-coordinate square planar geometry [24].
Two (d-d) transitions were shown in Figure 14 as explained below [Pt(HAS)2].5H2O .
1A1g → 1B1g (742 nm; 13477 cm-1)
1A1g → 1A2g (943 nm; 10604 cm-1 )
The peak at (443 nm; 22573 cm-1) was attributed to LMCT.
.png)
Thermo-gravimetric analysis (TGA)
TGA was used to investigate the temperature behavior of the synthesized ligand (HAS) and the complexes (Figures 15-19), while the number of stages changed. Calculated and discovered mass losses are given in Table 7. In our research, the weight loss followed up in the temperature range (25-1000) oC and subjected with argon flow. Thermal analysis is used to determine stoichiometry, thermal stability, and whether or not water molecules have crystallized or coordinated. The decomposed species were calculated using the weight losses for each constituent received from the thermal graph [25].
The results can be arranged as follows:
The results and the suggested formulae which is obtained from the analytical data seem to be identical.
- The thermogravimetric analysis data give an indication that the process of decomposition for the ligand (HAS) is carried out in several steps as well as their complexes.
- The thermal stability for HAS and its complexes was decreased as in the following order:
- HAS> [Ni(HAS)2(H2O)2].4H2O >[Co(HAS)2(H2O)2].4H2O > [Pd(HAS)2].H2O >[Pt(HAS)2 ].5H2O
Anti-oxidant and radical scavenging activity
Antioxidants help to stabilize a variety of goods, including meals, petrochemicals, medicines, and cosmetics and also help to strengthen an organism's defenses against free radical assaults. It can be found in both endogenous and exogenous forms in nature [26].
In vitro antioxidant of [Pt(HAS)2].5H2O was assessed using reductive ability and DPPH radical scavenging activity. In the reductive ability at all concentration (0.08, 0.16, 0.32, and 0.64) mg/mL for [Pt(HAS)2].5H2O was outperformed trolox (control) in the concentration-dependent reductive ability. It was (0.87167 ± 0.001528) at 0.08 mg/mL which was increased significantly to (1.66667 ± 0.005859) at 0.64 mg/mL (Table 8) indicating high reducibility for the complex when compared with trolox.
There is a lot of interest in creating a new form of synthetic chemical that is less hazardous and has no adverse effects. As a result of the current research using the DPPH test, the metal complex [Pt(HAS)2].5H2O was tested for its activity of scavenging. Figure 20 shows the scavenging effect of the tested chemicals at various doses, and it's clear that the action is reliant on coordination among the substances put to the test when compared to standard, the Pt (II) complex had good scavenging action, ascorbic acid used as control [1].
At the five concentrations which were examined (12.5, 25, 50, 100, and 200 mg/mL), [Pt(HAS)2].5H2O was substantially more active in DPPH radical scavenging activity than vitamin C. At concentrations of 12.5 mg/mL of [Pt(HAS)2].5H2O, it was (17.09± 1.857). At 200 mg/mL, DPPH radical scavenging activity increased dramatically to (72.62± 3.981). Table 9 and Figure 20 show the results.The survey in Anticancer Effectiveness
At the concentration (400 𝜇𝑔/mL), [Pt(HAS)2].5H2O had high anti-liver cancer activity. The cytotoxic efficacy was shown to be 60.65% efficient in killing tumor cells. Treatment of HepG-2 cells with [Pt(HAS)2].5H2O for 24 hours resulted in a significant rise in cellular metal (Pt) concentration compared to the control (WRL68), suggesting that the complex was easily internalized within 24 hours. MTT test may be used to calculate percentages of dead cells. The cytotoxicity of [Pt(HAS)2].5H2O is determined by the cytotoxic impact of Pt(II) conjugated in suppression of HDAC (Histon deacetylases) enzyme, according to the mechanical recommendation covalently binds the positive charge on the histone tail's N-terminal groups of lysine and the phosphor groups in DNA to have a negative charge. The covalent binding of Histone to DNA is critical for gene expression [27].
The half-maximum inhibitory concentration (IC50) is used to determine the efficacy of a medicine. IC50 for the tested substance is (108.3) g/mL during a 24 hours incubation period. As a result, fewer medications are needed to promote cancer cell death, and hence patients can tolerate them Table 10 and Figure 21 show the results after incubation with HepG-2 and WRL68 cells lines for 24 hours at concentrations of (6.25,12.50,25,50,100,200 and 400) 𝜇𝑔/mL of the [Pt(HAS)2].5H2O complex; modifications of morphological for DNA inclusiveness were performed. Chromatin condensation and nucleus fragmentation with a production of apoptosis and drug resistance were demonstrated by the presence of apoptotic bodies.
Antibacterial and antifungal activity for the synthesized ligand and the complexes
Metal (II) complexes are more efficient than free ligands, as indicated in Table 11, indicating that the reactivity of metal ions with the ligand is significant in increasing antibacterial activity. The membrane's lipid that surrounds the cell allows only lipid-soluble molecules to pass through, making lipo solubility an important component in bacterial activity regulation [29]. The examined compounds' method of action might include forming hydrogen bonds with infected cells' enzyme active sites via OH and/or N=N groups and effectively inhibiting them. Furthermore, the chemicals studied have the potential to disrupt organism respiration and impede protein synthesis. Variations in ribosomes in microbial cells or the impermeability of microbes' cells generate differences in the action of various complexes toward distinct species [30]. Finally, the ligand and complexes have effectiveness toward Rhizopus microspores fungi, especially [Ni(HAS)2(H2O)2].4H2O [31].
The activity increases in this order for (E.coli) bacteria : [Pt(HAS)2].5H2O > HAS > [Pd(HAS)2].H2O > [Co(HAS)2(H2O)2].4H2O > [Ni(HAS)2(H2O)2].4H2O
and for (Staph) bacteria ,the activity increases in this following order : [Pt(HAS)2].5H2O > [Ni(HAS)2(H2O)2].4H2O > HAS > [Pd(HAS)2].H2O > [Co(HAS)2(H2O)2].4H2O
while the activity for Rhizopus microsporus fungus increases in the following order : [Ni(HAS)2(H2O)2].4H2O > [Co(HAS)2(H2O)2].4H2O > and [Pd(HAS)2].H2O > HAS > [Pt(HAS)2].5H2O.
Burns healing (anti-inflammatory)
Due to their broad therapeutic usefulness, sulfonamides have become an attractive scaffold. The anti-inflammatory medicines like celecoxib, as well as the antibacterial agents such as sulfamethoxazole and sulfadiazine, are all sulfonamides that are routinely employed as therapeutic agents [32].
The ability of [Co(HAS)2(H2O)2].4H2O at (1.5) mM silver sulfadiazine (positive control) and negative control to heal burns was investigated by determining the number of days required to recover the results (Table 12), revealed that [Co(HAS)2(H2O)2].4H2O complex was able to repair burns in 10 days in comparison with what is shown in Figures 22 and 23.
Performance of dying
Wool fiber includes a variety of polar groups, including –NH, –OH, and–SH groups. An amino group at the end of the chain, as well as a significant number of –NH groups, may be found in polyamide fiber [33].
The type of metal and its valence state, the concentration of a solution, pH, duration, temperature, and other variables, all influence the pace and extent of absorption. The nitrogen atoms of amino and amide groups can form coordination bonds, especially at alkaline pH [34].
Figure 24 showed the wool textile dyeing with the ligand (HAS) and the metal complexes; the color of wool textile were at the range between orange, red and brown.
.png)
Wash fastness
Textile color fastness test for washing was carried out using soap with a concentration of 2%, and the results were very good (Table 13).
Acid - base indicator
Acid-base indicator is a chemical material used to determine if an aqueous solution is acidic, neutral, or alkaline in general. Because acidity and alkalinity affect pH, they are sometimes referred to as pH indicators, and their color changes as the pH changes. Azo dyes are one of the most widely utilized compounds as indicators because they have a squad of delocalization and bright hues, particularly yellow, red, and orange [35].
Conclusion
Based on the findings of spectral and analytical physicochemical investigations for the ligand (HAS) and its complexes, some conclusions have been achieved that lead to establishing the following points:
The ligand (HAS) was synthesized by the diazotization method with some modification and the ligand acted as neutral N, O-bidentate chelating ligand binds to the metal ions of interest. [Co(II), Ni(II), Pt(II), and Pd(II)], through the oxygen and nitrogen atoms of 7-Bromo 8-hydroxyquinoline, form a pentagonal chelating ring. Pt(II) and Pd(II) complexes are square planar, whereas Ni(II) complex is octahedral and Co(II) complex is distorted octahedral. A water molecule can be either coordination or crystalline. The synthesized complexes were characterized as high stability through examination of the stability constant and hot affected by moisture, light, and heat. Also through thermal analysis, the thermal stability of the produced compounds is shown by TGA. The ligand and the complexes have various anti-bacterial and anti-fungal activities. Some of the complexes are effective as anti-inflammatory, antioxidant, and anticancer. The ligand and the complexes can dye wool fabrics as they have different colors. The ligand (HAS) can change its color depending on the change in pH from acid to base which we can use it as an acid-base indicator.
Acknowledgements
This work was part of a research project of Duaa Jameel Jasim (M.Sc student) in (Science of Inorganic Chemistry, Department of Chemistry, College of Science, University of Baghdad, Baghdad, Iraq). And Supervised by Assistant professor Dr. Alyaa Khider Abbas
Orcid:
Alyaa Khider Abbas: http://orcid.org/0000-0002-8400-0926
------------------------------------------------------------------------
How to cite this article: Duaa Jameel Jasim, Alyaa khider Abbas. Synthesis, identification, antibacterial, medical and dying performance studies for azo-sulfamethoxazole metal complexes. Eurasian Chemical Communications, 2022, 4(1), 16-40. Link: http://www.echemcom.com/article_141627.html
------------------------------------------------------------------------
Copyright © 2022 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
