Document Type : Original Research Article
Authors
Department of Chemistry College of Education for Pure Sciences, Ibn–AL-Haitham University of Baghdad, Baghdad, Iraq
Abstract
The inhibitive effect of garlic extract on copper alloy corrosion in HNO3 acid solutions were studied by using weight loss methods. According to the findings, the extract was effective and very good inhibitors in the acidic media. In this study, the temperature influence was investigated on the corrosion efficacy in which there is (no) extract, and finally thermodynamic activation and adsorption parameters were determined. The results of every method used in this study showed a good agreement, We utilized the Langmuir adsorption isotherm to fit experimental data and the obtained adsorption Gibb’s free energy values and signs indicated the spontaneous adsorption of inhibitor molecules on copper surfaces by the mechanism of physical adsorption.
Graphical Abstract
Keywords
Main Subjects
Introduction
Corrosion naturally occurs affecting our life degradation to domestic gadgets and airplanes, distribution systems public roads, automobiles, and bridges [1]. Its key cause in metals is their tendency for reaching stable states. Many metal alloys are not stable and require interaction with the surrounding to obtain minor energies by the formation of the metal complexes [2].
The corrosions of the copper and copper alloys happen oxides layers (patina) are formed. Yet, these patinas degrade if exposed to polluted atmosphere [3]. Still, chemical inhibitor utilization like the chromate, nitrate, carbonate, phosphate, silicate, and other toxic compounds as an inhibitor of corrosion control confirmed their active inhibitors at relatively cheap costs, while these chemicals make additional obstacles not offering solutions [4].
Plant extracts are interesting because of the corrosion inhibitors for relatively longer periods appeared from the data in review papers. In particular, natural inhibitors are particularly interesting as they seem to be environmentally friendly, readily accessible, at low costs, with renewable supply sources [5–13]. The natural-happening substance includes many organic compounds which natural like ascorbic acid, flavonoids, and pigments. Their extracts are nitrogen, compounds in sulfur and oxygen making them active anti-corrosion inhibitors. In general, an inhibitor consists of heterogeneous atoms like (N, O, and S) possessing electronic densities which suit acting as antidotes to corrosion. N, O, and S are the adsorption active center on the metal after the inhibitor of the competences of P>S>N>O [14].
Garlic is vegetable with a bulb of the family Liliaceae and is commonly distributed worldwide and China is the leader with about 81% of world. It is one of the significant preventive herbs, a spice, and a well-trusted remedy in different epidemics like dysentery, typhoid, cholera, and influenza. It is a remedy different ailment. Garlic has at least 100 sulphur-containing compounds basically to medicinal applications. Allicin is 70 to 80 % of the total thiosulphinates in it. Its smell is slight and imperceptible until peeled. Upon peeling, slicing or crushing, it at once spread intense smell with sulphur glycosides. Several studies revealed that allicin is a significant garlic element responsible for its odours, flavor and its biological features.
Although it is high medicinal and culinary values, garlic has anti-nutrition like flavonoids, saponins, tannins, alkaloids, steroids, hydrocyanide, and anthocyanin. Flavonoids, saponins and tannin of the garlic are in 0.04 to 0.36%, 0.14 to 19.0%, and 0.06 to 6.10%, respectively [15].
In this article, the new environmentally friendly corrosion inhibitor effects (garlic extract) like corrosion inhibitors to copper in nitric acid solutions were studied by the methods of the weight loss.
Experimental
Materials
The utilized copper sheet has a purity of >99.% (supplied by Local Market in Iraq). This sheet cut the samples (dimensions= 2 cm diameter= 0.5 cm). It is also mechanically polished with grade emery papers 220, 400, and 600. In addition, it is washed exhaustively with distilled water, while washing with ethanol and acetone to degrease it, and then it was stored in desiccator prior to their uses in corrosion examinations.
Sample collection and preparation
Garlic was taken from Local Market in Iraq. Distilled water was used to clean and wash it, and then it was dried at room temperatures and grounded to be powdered. Then, it was stored in the dark within containers of glasses for additional used (50 g) of it. It was put after drying in 500 mL round bottomed flasks with a 400 mL of 0.5M HNO3 and refluxed for 1 hour in boiling degrees. Next, it was left to cool at room temperatures out of the light. The mixture was filtered with filter papers at various concentrations (200, 250, 300, 350, and 400) ppm of stock solution.
Quantification of all alkaloids, saponins, and flavonoids content in the garlic extracts
Garlic powder and quantification chemical compositions of all .alkaloids, saponins, and flavonoids were made based on the standards (Fajemileh in Samuel Oladipo Kolawole) chemical compositions, phytochemical, and mineral profiles of garlic (Allium sativum), 2018 [15].
The weight loss approaches the easiest to study corrosion inhibitors because devices (except for the use of the digital scale) are needed. This approach has the weight metal sample differences of the measured pre- and post-exposing the corrosion media (with and without inhibitors). This work weighed copper specimens completely immersing it in 0.5 M HNO3 solutions with no (control samples) and with the garlic extract. In terms of specimens, they were circular copper foils (dimensions: 2 cm diameter and 0.2 cm thickness). We conducted these experiments at various concentrations of garlic extract: (200, 250, 300, 350, and 400) ppm. All weight-loss calculations re-occurred three times followed by the comparison of the results. The test lasted 2 hours in stirred settings at 25 °C (298 K) in the acid media by using a new fresh solution in all experiments.
The inhibition competence (%IE), surface coverage values (𝜽), and corrosion rates were measured as follow:
%IE=[(CRo-CRi)/CRo]×100% (1)
𝜽=(CRo-CRi)/CRo (2)
CR(mdd)=(wo-wi)/A×t (3)
Where, wo and wi are copper weight losses in mg with and without inhibitors, respectively, 𝜽 is the surface coverage of inhibitors; A in the outer layer areas of the copper specimens (in dm2), and t is immersion times (in days)[16].
Results and discussion
Concentration and temperature influence
The inhibition efficiency differences were resulted due to the weight losses in other inhibitor concentrations in 0.5 M HNO3 at variable temperatures (298, 308, 318, and 328K) appear in Table 2.
Based on the results, the inhibition efficiency IE% increases when concentration of the inhibitors rises and is declined by the reduction of temperatures at the same inhibitor concentrations, the inhibition efficiency showed an inverse proportion to the temperatures in which highest efficiency reached 87.9% at (298) K [18] because the adsorption amounts and the inhibitor molecule coverage on copper outer layers rises with the inhibitor concentrations, so copper surfaces are professionally drifted from the medium decrease in inhibition efficiencies with rise of temperatures suggesting physical adsorption mechanism[17,18].
Copper corrosion activating energy:
The activating energy measurements are for the corrosion coppers in nitric acids with (without) no garlic extract that are evaluated from Arrhenius Equation [19,20]:
Where, (Ea) and (R) are the activating energy is the gas constant, respectively (8.314), while (A) refers to the Arrhenius constant. According to Equation 4, plotting log(icorr) versus 1/T should appear linear as we practically noticed line slopes giving= -E*/RT , yet the line intercept extrapolated to 1/T=o giving ln A.
By using alternative Arrhenius relationships (∆H) and (∆S):
Hence, (h) is "planks constant"(6.626*10-34 J.S), (N) is "Avogadro's number" (6.022*1023 mol-1), (In icorr/T) vs. (1/T) ( can be drawn. Here, a straight line slope depicts its values (-∆Hact/R) and the intersections reveal it values (In R/Nh+∆Sact/R) , as indicated in Figure 1 and Table 3.
The rise of the Arhinius coefficient becomes stable showing high reaction rates, yet activation energy reduction reveals the Arhinius coefficient stability in a low reaction rates. The activating energy variations and the Arhinius coefficient are clear in which reacting corrosion starts with sites showing low activating cards spreading to higher activation energy sites [21].
So, adding inhibitors raises the energy barriers of copper corrosions in the solutions with chlorine ions, while corrosions show and that the inhibitors postpone it. Also, temperature increase raises the corrosion and then decreased the inhibition efficiency in which rises in inhibitor concentration increases the densities of electrons in the adsorption centers of the molecules of the inhibitor improving the inhibition efficiency [22].
The adsorption isotherm
The surface coverage data is important in evaluating the inhibitor features and it is valuable in the discussion of the adsorption features with the isotherm adsorption help as adding inhibitor molecules on the surfaces in the metal interaction with them.
The examination of the outer coverage (𝜃) of the operating electrode (copper) surfaces through the inhibitors (garlic extract) and the concentrations of inhibitos’ solutions (𝐶𝑖𝑛ℎ) are conducted fitting different adsorption isotherms. The best was gotten with Langmuir isotherm given by the next equation [23,24].
Where, Kads is the equilibrium constant of the adsorption/desorptionreflecting that theinhibitor molecules are approachingthe surface adsorption sites.
The measured Kads, the Gibbs free energies of adsorption were measured by [25].
In which, R is gas constants, T is the absolute temperatures and the values (55.5) are the molar water concentrations in mol/L, while the standard enthalpies of adsorption are measured by the van ҆t Hoff formula;
Plots of in Kads values vs. 1/T values showed the straight lines slope equal -∆Hact/R. The negative values revealed adsorption of garlic extract molecules as exothermic. The Δ𝐺°𝑎𝑑𝑠negative values with the inhibitor are followed by exothermic adsorption processes. Δ𝑆°𝑎𝑑𝑠 of inhibitions are measured from [26] as in Equation 4:
The above results shown in Table 4.
ΔGads values reached -20kJ/mol were considered as the electrostatic of the charged molecules interacting with the metal outer layers (physisorption). Yet, the ΔGads values are more negative than -40kJ/mol as charges in common or transferring from the molecules of the inhibitors to the metal surfaces to form coordinating covalent bonds (chemisorption). The ΔGads values ranged (12-14) kJ.mole-1, meaning the inhibitor adsorption on copper alloy surfaces happen by physical adsorptions [27].
The negative values of (∆H∘) indicate an adsorption of the inhibitor molecules as exothermic. The adsorption could be either chemical or physical, yet the positive value shows an endothermic aspect attributed to chemisorption. Thus, the adsorption of the garlic extracts molecules on the copper surfaces refer for physisorption in this article [28].
Scanning electron microscope (SEM) measurements
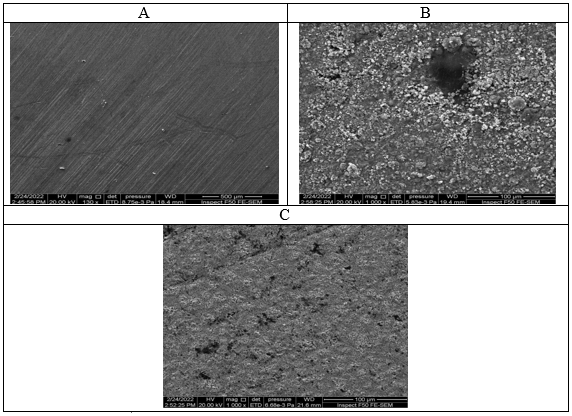
Surface of copper were analyzed by SEM to verify the absorption of the extracted molecules as adsorbed on the surfaces of the copper alloys or just peeled off the surfaces. The SEM micrograph for copper alloy surfaces following their immersing in 0.5 M of nitric acid adding or not adding the ideal concentrations of garlic extract as in Figure 4 in which in Figure a is polished alloys of copper, (b) copper alloys immersed in nitric acids (HNO3), and (c) copper alloys in (0.5) of M(HNO3) solutions with a 400 ppm of garlic extracts comparing the SEM micrographs with and without the extract shows the great inhibitive effects of those compounds.
Conclusion
The results of this study indicated that garlic extract an efficient inhibitor for copper in 1M ( HNO3) solutions by using weight loss techniques. The negative values of ΔG reveal that the adsorption of the inhibitors on the metal surface is spontaneous. Furthermore, Ea and ΔH values of the corrosion process support this observation. All values of ΔS*ads are negative for the blank and inhibited solution which implies that the activation complex in the rate determining step represents association rather than dissociation step. The adsorption characteristics of garlic extract on the metal surface were approximated by Langmuir adsorption isotherm, and it obeyed and fitted this model. The protection efficiency was enhanced with the increase of inhibitor concentration. The highest value reached 87.9% at 400 ppm of garlic extract.
Acknowledgements
The authors extend their acknowledgment to the Deanship of College of Education for Pure ScienceIbn-Al-Haithamat University of Baghdad for supporting this research.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this manuscript.
Orcid:
Nawras Saad Mohamed Ramadan: https://www.orcid.org/0000-0001-7780-4048
Zainab Wajdi Ahmed: https://www.orcid.org/0000-0001-8267-6212
----------------------------------------------------------------------------------------
How to cite this article: Nawras Saad Mohamed Ramadan*, Zainab Wajdi Ahmed. Effect of garlic extract as corrosion inhibitor for copper in acidic medium. Eurasian Chemical Communications, 2023, 5(1), 28-36. Link: http://www.echemcom.com/article_154675.html
----------------------------------------------------------------------------------------
Copyright © 2023 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
