Document Type : Original Research Article
Authors
Department of Chemistry, College of Arts and Science, University of Benghazi, Al kufra, Libya
Abstract
Alkaline earth metal oxides (CaO–MgO) are employed as a heterogeneous catalyst in the manufacture of biodiesel from non-edible oils such as olive, sunflower, and corn. The purpose of this research is to examine the effect of temperatures on the catalytic conversion of biodiesel from waste cooking oil using CaO–MgO. The catalyst was used to investigate the temperatures effect on the trans-esterification reaction. The best circumstances were studied a methanol to oil ratio of 6:1 and temperatures of 30-60 °C. The temperatures of the reaction has a considerable influence on the trans-esterification reaction, with optimum biodiesel conversion occurring at 60 °C and a yield 99%, with R2 values larger than 0.70. CaO–MgO catalyst exhibited 99% yield and strong catalytic activity. According to the experimental results, the CaO–MgO heterogeneous catalyst can obtain biodiesel of high yields that are comparable to those cited in the citations.
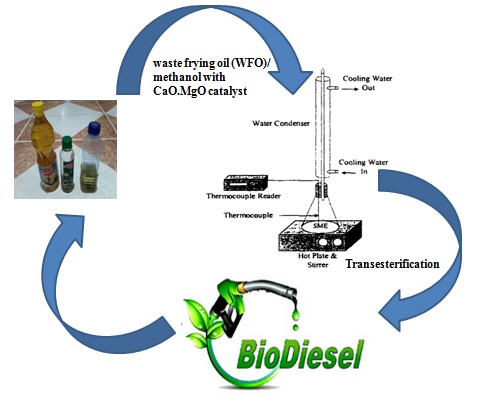
Graphical Abstract
Keywords
Main Subjects
Introduction
The global need for energy is rising due to expanding industrialization and population growth, which is increasing the consumption of fossil fuels. Since the 1970s, the price of mineral-based fuels like petroleum, natural gas, and diesel has increased due to the rapid consumption and depletion of these fossil fuels. Substitute fuel has drawn a lot of interest due to the rise in greenhouse gas emissions and the depletion of fossil resources. Many developed countries are showing increasing interest in leveraging new knowledge and diverse biofuels to produce bio-energy that is less expensive than fossil fuels [1,2]. In this regard, fatty acid methyl esters (biodiesel fuel) derived from the trans-esterification of vegetable oils and animal fat (edible and non-edible plant oils, fungi, and animal fats) have drawn a lot of attention recently as sustainable and environmentally friendly, biodegradable, free of sulfur, and aromatic compounds, and safe fuels [2].Due to the many sources of the oils, a classification system for the oils will be needed. This system should be based on the evaluation of objective criteria connected to the hazardous compounds present in oils. The two main categories where these chemicals fall are oil decomposition compounds and fat-soluble pollutants such as dioxins and substances that are similar to dioxins, polycyclic aromatic hydrocarbons (PAHs), and polychlorinated biphenyls (PCBs). It has been discovered that the decomposition of oil produces toxic PCBs, dioxins, and dioxin-like compounds [3,4]. Kitchen waste, also known as non-edible waste, develops as a result of the population's increasing demand for consumables like cooking oil and renewable hydrocarbon fuels (RHFs), also known as agro-food industries (AFI) [3]. Whether frying meals at home, in restaurants, or in the food industry, cooking oil is one of the most crucial ingredients in food preparation. The frying process causes the oil to go through a lot of physical and chemical changes. Long-term frying causes these changes, rendering the oil unfit for consumption. They include physical changes in color, odor, viscosity, and caloric content, in addition to chemical changes caused by the presence of particulate matter, increased levels of total polar solids, and polymeric particles as a result of chemical interactions [2,3,4,5,6,7,8].The process of trans-esterifying a variety of renewable resources, including animal fats and edible vegetable oils including palm, sunflower, rapeseed, cottonseed, soybean, and algal oil produces a mixture of alkyl esters known as biodiesel. It may be utilized in diesel engines with very little modifications because its properties are essentially equal to those of petro-derived diesel. Consequently, biodiesel is defined as a mixture of long chain fatty acid methyl (FAME) or fatty acid ethyl esters (FAEE) produced by the esterification and trans-esterification reactions of free fatty acids (FFA) and triglycerides (TGs) that naturally occur in renewable biological sources such as vegetable oils and animal fats with short-chain alcohols [9,10,11]. In addition, it produces fewer harmful pollutants than conventional petro-diesel and is non-toxic and biodegradable. Nevertheless, the high cost of resources is responsible for around 88% of the overall cost of producing biodiesel. Thus, non-edible oil feedstock for biodiesel production has attracted a lot of attention in recent years. Examples include used cooking oil, natural fat, jatropha oil, used grease, and microalgae. This feedstock are challenging to manage because they typically contain a lot of water and free fatty acids (FFA), which necessitate pretreatment to achieve conversion efficiencies that are economically acceptable when a suitable catalyst is present [9,10,11,12]. The choice of the catalyst, which determines the cost of production and creates the economic obstacle, is a crucial step in the trans-esterification process. In the trans-esterification reaction, the catalyst is the kingpin, as displayed in Figure 2. Chemically, the major difference between biodiesel and traditional petro-diesel fuel molecule is the presence of an ester group in biodiesel molecule, as depicted in Figure 1. This ester group makes biodiesel an oxygenated and cleaner fuel (high oxygen content and low emissions), as compared with diesel fuel.
Biodiesel was produced using alcoholysis or trans-esterification reactions with a base, acid, enzyme, and other catalysts. Both chemical and biological catalysts have advantages and disadvantages which are being investigated [12].According to reports, these catalysts are economical and budget-friendly materials for industrial applications. Catalysts used in chemical reactions might be homogeneous (acid or alkali), heterogeneous (solid alkali or acid catalyst), supercritical fluids (SCF), or heterogeneous nanostructured. Several main alcohols, including methanol, ethanol, propanol, and butanol can be utilized in the trans-esterification since they are inexpensive [12]. Methanol is typically utilized in trans-esterification because of its physical and chemical properties [10].
Catalysts are essential components in the production of pure and high-quality biodiesel. The manufacture of biodiesel can be complicated by homogeneous catalysts, which can cause saponification of the feedstock and produce large quantities of unwanted byproducts like soap. This prevents the splitting of the fatty acid methyl esters (FAME) and glycerol and decreases the catalyst. Although homogeneous catalyst trans-esterification is simple and rapid, it has disadvantages regarding catalyst separation, reusability, and renewable resources. In recent years [13,14,15,16,17,18,19], heterogeneous catalysts with appealing characteristics for petro-diesel production have been regarded as environmentally benign catalysts. Heterogeneous catalysts have piqued the interest of researchers due to their competitive advantages over homogeneous catalysts, which include regeneration, facile product separation, recyclability, high selectivity, biodegradability, and no reactor corrosion [20,21,22,23,24]. Heterogeneous catalysts capable of accelerating trans-esterification [25,26,27,28,29] are particularly appealing. Basic catalysts exhibit high trans-esterification activity rates at low temperatures. Catalysts of CaO–MgO were utilized to evaluate how the catalyst affected product yield. They observed a significant amount of residual oil was created as a result of the basic centers’ ability to stop secondary cracking. The goal of the current study is to assess the viability of producing biodiesel from certain types of cooking oil waste utilizing heterogeneous catalytic methods. Based on the advantageous traits of catalysts and the significance of non-noble metal promoters in the trans-esterification process, this work aims to achieve a high biodiesel yield supported on inorganic metal oxides. Strong basic characteristics are proven to be necessary for this reaction across various catalytic systems used in the trans-esterification of waste feedstock using basic catalysts. Systems in the form of a simple oxide display some intriguing characteristics.
Materials and methods
Materials
The olive, sunflower, and corn oils were purchased from local markets in Kufra Municipality, southeast Libya. Commercial alkaline earth metals, such as calcium hydroxide (Ca(OH)2, 99.995%) and magnesium hydroxide (Mg(OH)2, 95%) were bought from Sigma-Aldrich. Methanol (CH3OH, 99.9%) reagent was purchased from Thermo Scientific™.
Experimental methods
Synthesis of alkaline earth metal oxide catalyst as (CaO–MgO)
The commercial samples of magnesium hydroxide (Mg(OH)2) and calcium hydroxide (Ca(OH)2) were thermally decomposed at 600 °C to create the catalyst samples. As an illustration, 1 gm of Ca(OH)2 and 2 gm of Mg(OH)2 were combined to form the sample, which was then dissolved in 20 ml of distilled water. Afterwards, the precipitate was recovered by filtering and dried in an oven at 300 °C for 4 hours to eliminate excess water. This produced Ca(OH)2–Mg(OH)2. This phase is known as the hydration strategy. The dried Ca(OH)2–Mg(OH)2 was then pulverized into a fine powder by using a mortar and pestle. The subsequent stage, the dehydration procedure, was carried out to promote the formation of the oxide CaO–MgO by calcinations in a furnace at 600 °C for two hours. Following that, silica gel pellet-filled desiccators were used to store the sample as a catalyst in glass bottles to prevent activity degradation.
Procedure
Two-step reaction
In the two-step process, the first step, Fischer esterification involves the conversion of free fatty acids with methanol (as an acid catalyst), into their corresponding fatty acid esters. The second step, trans-esterification, involves the conversion of triglycerides with methanol (in the presence of an alkaline earth metals catalyst) into their corresponding fatty acid esters.
Conversion of oil to esters (esterification and trans-esterification)
The non-edible (olive, sunflower, and corn) oils were collected and filtered and heated at 110 °C for 30 minutes to eliminate any solid contaminants and water molecules. This step is required to be performed prior to starting the trans-esterification process. This process only took around 30 minutes. Prior to trans-esterification, the high acid value brought on by the presence of unesterified fatty acids was reduced using the acid catalyst process known as esterification. Thus, the reaction was carried out in a 250 ml round bottom flask equipped with a reflux condenser and an agitated stirrer. At this stage, the non-edible oil was poured into the three-neck flask, and methanol was added at a 6:1 methanol-oil molar ratio, and then they were mixed thoroughly to form a homogenous mixture. In addition, the esterification product cooled at room temperature before being moved into a second funnel within 24 hours to complete the separation process. The upper liquid, which was removed and presumed to be triglyceride product, was employed as feedstock for the trans-esterification procedure. The following steps were taken during the trans-esterification process: 2 weight percent of CaO–MgO catalyst was added to the methanol while it was being stirred, and all components were mixed together to maintain reaction temperatures. The oil obtained from the esterification process added into the mixture and heated at different temperatures for four hours under the stirring process at a fixed speed of 400 rpm. The samples were centrifuged for 10 minutes to separate the upper layer of liquid from the lower layer of solid. The glycerol layer, FAME layer, and methanol layer made formed the topmost layer, from bottom to top. The trans-esterification product was placed into a separate funnel and kept for 24 hours at room temperature before being entirely partitioned into three phases. While glycerol and the leftover methanol made up the bottom layer, the top layer of the liquid phase was removed as crude biodiesel (FAME).The FAME layer was gathered to establish the yield content. Since alcohol and triglycerides are incompatible at room temperature, stirring is necessary to improve phase contact and ensure complete mixing while avoiding mass transfer constraints. Two steps are needed to be taken into consideration: The reaction mixture moves from the zone that controls mass transfer (a heterogeneous system where methanol is the dispersed phase and oil is the continuous phase) to the region that controls kinetics (pseudo-homogeneous system in a single phase).A highly rapid emulsion is created when methanol is used, and it immediately separates into two phases: an upper phase made of methyl esters and a lower phase made of glycerol. When CaO–MgO is utilized as a catalyst, it dissolves in the alcohol before triglycerides disperse through the mixture, controlling the reaction at first by mass transfer. In these circumstances, the alcohol-to-oil molar ratio ranges from 6 to 1, this is fairly high. Alcohol serves as an acid catalyst under these circumstances, and the presence of FFA and water in the feed has no impact on the trans-esterification conversion. CaO–MgO catalyst that improves phase contact can be employed to reduce mass transfer limits even more. This so-called heterogeneous catalyst, CaO–MgO, falls under that category.
Results and discussion
The production and usage of biodiesel have increased dramatically in the twenty-first century due to the benefits associated with its ability to reduce ozone-depleting substances. A stable catalyst in terms of catalytic life, recyclability, and lower cost are crucial as these directly affect the entire cost of the process. The heterogeneous strong catalysis options were considered for the specific research results of trans-esterification on heterogeneous catalysis, which mostly focused on investigating the appropriate oil supply, methanol to oil molar proportion, and measuring the accessibility of catalyst. The fundamental sites were divided into three categories: strong, super, and moderate. The strong sites were attributed to Ca2+–O2– species, the super basic to isolated O2– sites, and the moderately basic to Mg2+–O2– species. In addition, the catalytic activity would rise if the catalyst's temperature were raised from 30 to 60 °C. This was attributed to the incomplete decomposition of the hydroxide compounds that provided the active sites and remained at 60 °C. At higher temperatures, the complete formed oxide species would agglomerate and lose crystalline phases and active sites. These samples reached a biofuels yield of 99% using 2% of catalysts with a methanol: oil molar ratio of 6:1 at 60 °C for 4 hours. As a result, the O2- produced by the CaO–MgO catalyst would remove the H+ from water to produce a surface-level OH- group, which may then remove the H+ from methanol to form a methoxide anion (CH3O−), (Figure 3). Due to its alkaline nature, the methoxide anion is regarded as being extremely active in the trans-esterification processes. As a result, the CH3O− group attacks the carbonyl group of a triglyceride (TG) to produce a tetrahedral intermediate that will accept the adsorbed H+ group from CaO–MgO. The intermediate will finally undergo a self-rearrangement to yield FAME molecules and glycerol (Figure 4). Therefore, if we assume that there is water involved in the reaction. It was shown that water could facilitate the ionization of methoxide (Figure 3).To achieve 99% production and conversion of biodiesel, CaO–MgO was employed. It was found that the preparation process, reaction duration, temperature, and oil to methanol molar ratio significantly affect biodiesel production.
Chemically, all vegetable oils and fats, regardless of different flavor and color, are remarkably similar. These are mixtures of fatty acid esters of glycerol and are known as triglycerides. Triglycerides are formed by the esterification of all three hydroxyl groups of the glycerol by saturated or unsaturated (C13 to C21) long straight-chain fatty acids. Two explanations were proposed to explain this behavior of alcohol. First, the relative smaller size of the methanol molecule could facilitate the simultaneous attack of CH3OH on all three chains of the triglyceride. Second, the greater polarity of the methoxy anion might promote its attacking ability on the carbonyl group of ester.
Figure 5 displays a graph of the biodiesel (FAME yield %) as a function of reaction temperature. This graph makes it evident that the conversion increased at the initial temperature, and then slightly increased as the reaction temperature increased higher. At 30, 40, 50, and 60 °C, the reaction temperature for yield was measured (Table 1). The other reaction parameters were kept at 6:1, a 2 weight percent catalyst concentration, and a 4-hour reaction period. At a reaction temperature of 60 °C, the greatest conversion was recorded at 99%. It was discovered that the biodiesel output was marginally declining at 30 °C. It can be inferred that the feasible reaction temperature for transesterification over CaO–MgO catalyst is 30-60 °C, because higher reaction temperatures may produce substantial changes in FAME yield for biodiesel generation. The graph clearly shows that the process for biodiesel generation began at 30 °C and increased in FAME yield until it reached 60 °C. At this point, the greatest FAME yield was obtained, and this yield stability was seen up to 60 °C. At 30 °C, there was a slightly drop in FAME output (Sunflower oil, 76%) (Table 1). The higher temperature of transesterification reaction increased the yield of biodiesel to some amount. Furthermore, the findings were compared with citations for trans-esterification of several oils with methanol to evaluate how reaction temperature influences the outcomes (Tables 1 and 2). As it can be seen in Table 2, the increased temperature resulted in a significant increase in biodiesel production rate, while response time had no effect. In contrast, Microsoft Excel was used to compute the correlation coefficient (R2). R2 should be near 1.0 for a statistical model to be useful. The results were comparatively strong and quite high at (sunflower oil R2= 0.994, corn oil R2= 0.98), although olive oil (R2 = 0.727) suffered a decrease in value.
Conclusion
Globally, there is a rapid increase in demand for sustainable and renewable fuels due to increase environmental pollution from petroleum fuels and decrease fossil fuel resources. Biodiesel has grown in popularity as a fuel in recent years due to its environmental benefits and manufacture from renewable feedstocks. Despite the fact that the price of crude oil directly influences the development of biodieel technology, many research initiatives in this field are still subject to change. In a system that generates biodiesel at a low cost, selecting an adequate source of the fuel is essential because feedstocks account for the majority of the cost of making biodiesel. Focusing on feedstock that does not impede food production and does not require land clearing, and also reducing greenhouse gas emissions is crucial when looking for practical biodiesel sources.
Acknowledgements
The authors are grateful to Mohamed Saleh Korbag for his language review of this article and for taking the time to provide valuable feedback.
Conflict of Interest
The authors declare that they have no conflict of interest.
Orcid:
Salma Korbag: https://www.orcid.org/0000-0001-7493-0698
---------------------------------------------------------------------
How to cite this article: Salma Korbag, Issa Korbag. Trans-esterification of non-edible oil with a CaO-MgO heterogeneous catalyst to produce biodiesel. Eurasian Chemical Communications, 2023, 5(5), 382-391. Link: https://www.echemcom.com/article_164834.html
---------------------------------------------------------------------
Copyright © 2023 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
