Document Type : Original Research Article
Authors
Department of Chemistry, College of Science for Women, University of Babylon, Hilla, Iraq
Abstract
In this article, a Schiff base ligand was produced with Co (II) and Ni (II) complexes. The Schiff base was prepared by the condensation reaction between 9,10-phenanthrynquinone and 2-mercaptoaniline in a ratio of 1:1, respectively, and the reaction time was three hours. While the Co (II) and Ni (II) complexes were prepared by reacting the prepared Schiff base ligand with the metal chloride salts in ethanol also in 1:1 molar ratio of L:M and the synthesis time 12 hours. The produced ligand was characterized by infrared spectroscopy, UV-Vis, 1H-MNR, mass spectroscopy, and thermal gravimetric analysis, while the complexes were also characterized by UV-Vis spectroscopy, FTIR, conductivity measurements, atomic absorption, magnetic susceptibility, and thermal gravimetric analysis. The resulted complexes were green in color. Based on the results, the coordination between the metal ions and the Schiff base occurs through the imine group of the Schiff base ligand, the thione group and the remaining oxygen atom of 9,10-phenanthrynquinone. While the conductivity test shows that the two complexes are non-electrolyte in nature where ethanol was used as a solvent. The magnetic susceptibility values of Co and Ni (II) complexes were found to be 3.09 and 3.3 7 BM, respectively which indicate the presence of three unpaired electrons and two unpaired electrons in both complexes, respectively. These values also support the octahedral geometry of the higher spin complexes and confirm the sp3d2 metal hybridization in the complexes. The atomic absorption values of the complexes and the thermogravimetric analysis confirm that the proposed structure for the complexes is [M(L)ClH2O)]XH2O.
Graphical Abstract
Keywords
Main Subjects
Introduction
A substance having a functional group that has a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or an alkyl group rather than to hydrogen is known as a Schiff base [1-2] . The chemical structure of this kind of bases contains the interesting imine (-C=N-) functional group. Due to the presence of this group, Schiff bases have been applied in different biological and industrial fields. For example, in biological applications Schiff bases have used as antifungal, antibacterial, anticancer, and antioxidant in coordination chemistry because of their variety of ways where they can attach to metal ions, their stability, and their biological consequences, Schiff bases have been widely used as ligands. Due to their strong thermal stability, high conductivity, and favorable luminescence characteristics, metal complexes of the Schiff base have received a lot of attention in recent years. In general, the imine nitrogen and aldehyde group are connected to Schiff-base ligands coupled with metals. The aromatic bridging azo methine metal's luminescence properties [3-4].
In addition, the structure of these ligands with several donor atoms, such as N, O, S, and others, are particularly interesting especially in biology [5]. Due to their strong thermal stability, high conductivity, and favorable luminescence characteristics, metal complexes of the Schiff base have expected a lot of attention in new years. In general, the imine nitrogen atom is connected to with metal [6].
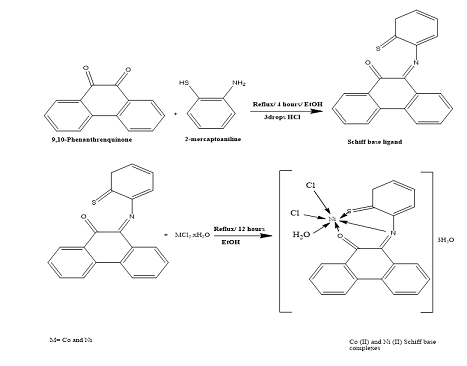
In the work a Schiff base, ligand was prepared form the reaction between 9,10- phenanthrenquinone with 2-mercaptoaniline and Co (II) and Ni (II) complexes with this Schiff base ligand were prepared and characterized.
Experimental
Chemicals
9,10- Phenanthrenquinone and 2-mercaptoaniline ware purchased form Macklin Bio-Chemical Co. Metal chlorides were purchased form Merck and C.D.H. Ethanol was ECOCHEM. Hydrochloric acid was supplied from Thoms Baker for chemicals.
Instruments
Using a KBr disk and a Fourier transform infrared spectrophotometer (FT-IR-8400S) from Shimadzo, the infrared spectra were measured. Using a UV-Visible spectrophotometer (PEAK INSTRUMENTS/ C-7200) from Shimadzo the electronic transitions were measured. The nuclear magnetic resonance spectra were measured using Bruker spectrometer Operating at (400Hz) with (DMSO). The differential thermal gravimeter (DTG-160-FC-60A) was used to measure thermal analysis, and the WTW SERIES, cond 722 was used to test conductivity. The Sherwood Scientific Auto Balance Magnetic Susceptibility Balance was used to measure magnetic susceptibility. Melting point/SMP30Stuart device was used to measure melting points. A SMP30 Stuart melting point apparatus was used Mass measurement in Iran Mashhad University of Medical Sciences.
Synthesis of Schiff base ligand
Three drops of hydrochloric acid were added to a mixture of 9,10 (1 g, 4.803 mmol) and 2-mercaptoaniline (0.602 g, 4.7 mmol) in 25 ml of absolute ethanol. After three hours of mixture reflux a green Schiff base ligand was precipitated which was then washed with water and ethanol and crystallized from the ethanol.
Synthesis of cobalt (II) and nickel (II) complexes
The two metal complexes were prepared by dissolving the prepared Schiff base ligand (0.5 g, 0.95 mmol) in 25 mL ethanol and mixing it with CoCl2.6H2O.(0.337 g, 1.598 mmol), NiCl2.6H2O (0.337 g, 1.59 mmol) in 1:1 L:M molar ratio. The mixture was refluxed for 12 hours. Also green precipitates were formed for Co (II) and Ni (II) complexes which were filtered, washed with water and ethanol
Results and discussion
Table 1 presents some of physical properties of the ligand and the complexes and Scheme 1 steps to ligand synthesis and its metal complexes.
Mass spectra
It is successful to explore molecular weight of the ligand using mass spectrometry. By this technique, certain peaks appear which match to different fragments. These peaks' intensities provide information on the stability of fragments (supplementary material). The molecular weight of the ligand in the theoretical calculation is 315 g/mol and the practical value from the mass measurement is 314 g/mol [7].
1H-NMR spectra
The Schiff base ligand was characterized by 1H-NMR spectroscopy using DMSO-d6 as the solvent where its protons were identified at 2.5 ppm. In addition, this 1H NMR spectrum exhibits aromatic proton-related signals in the region of 6.5-9 ppm. Moreover, a signal between 12 and 13 ppm in the ligand structures' S-H bonds was found [8,9]. Scheme 2 shows 1H-NMR spectra of the ligand.
FT-IR spectroscopy
The Schiff base ligand's FT-IR spectra reveal distinctive new peaks which show the formation of this compound. For example, at 1516 cm-1 a new peak was identified as attributed to the imino group (C=N) of Schiff base ligand. The absence of an NH2 absorption band in free 2-mercaptoaniline at 3446-3356 cm-1 along with the absence of a (C=O) group adsorption band in 9,10-phenathrenquinon at 1674 cm-1 further supported the creation of this group. The ligand spectra also indicates that the 2-mercaptoaniline 's (S-H) vibration frequency, which is present at 2522 cm-1 as a single band, has been converted into thione form (C=S) at 1323 cm-1 [10]. When the metal complexes are produced, the two bands (C=N) and (C=S) are shifted and changed. The two metal complexes show a shift in the (C=N) frequency to 1508 cm-1, indicating that the metal coordinates with the ligand through nitrogen of the imino group. While the (C=S) was moved to 1338 cm-1 in the Co2+and Ni2+, complexes [11], suggesting that thione group is coordinating with the metals. The free carbonyl group in the ligand structure is another coordination site with the metals also. Where this group is shifted from 1674 cm-1 to 1670 cm-1 in both complexes. Likewise, FT-IR spectra of both complexes show bands between the regions of 3200-3600 which indicate the presence of hydrated water molecules.
M-N, M-O and M-S bonds are represented by the new bands that develop at 400-600 cm-1. While a broad peak in the complex chemical structure refers to the presence of hydrated water. Table 2 presents the characteristic bands in the FTIR spectrum of the ligand and how they were shifted upon complex formation.
Electronic spectra
The electronic spectra (Scheme 3) of the prepared compounds were measured in the range of (200-900) nm at room temperature using ethanol as a solvent [12]. In Figure 3, the ligand spectra shows two bands at 235 and 423 which are related to π-π* and n-π*, respectively. While the spectra of [Co(L)Cl2H2O]3H2O shows peaks at 295, 370, 427, and 628 nm refer to the INCT, 4T1g→4T2g, and 4T1g→ 4A2g transitions, and also [Ni(L)Cl2H2O]3H2O shows transitions at 292, 327, 433, and 635 which represent INCT, 3A2g →3T1g(P), and 3A2g →3T1g(F) in the octahedral complexes [13]. Table 3 illustrates the values of electronic transitions in the UV-Vis spectra of the ligand and Co (II) and Ni (II) complexes.
Molar conductance measurements and atomic absorption
Ethanol was used to measure the conductivity of the formed complexes at a concentration of 1x10-3 M. Table (4) shows that the two complexes are non- electrolytes [14,15]. And these results also confirmed the presence of the two chloride ions inside the coordination sphere. In addition, Table 4 indicates the obtained metal content in both complexes which agrees with the theoretical values and this also confirms the 1:1 molar ratio of M:L.
Magnetic susceptibility
It is apparent from the magnetic susceptibility values in Table (5) that the Co(II) complex (3.09 B.M) is paramagnetic and that it is available as a high spin complex with a +2 oxidation state of the central ion with three unpaired electrons in d-orbitals. This result supports geometry of Co(II) complex in which is octahedral [16]. While the nickel (II) complex has a magnetic susceptibility value of 3.37 BM, which shows that it is also paramagnetic with two unpaired electrons in d-orbitals. It’s octahedral in shape [17-18].
Thermal analysis
Thermal analysis took place between at 0-800 °C under air. The loss of water at 100-150 °C has a thermal analysis of the complex [19]. Table 6 shows the breakdown temperature range and mass for all the chemicals under study. In Figure 4, the TG technique includes the ligand decomposition in two steps, the first through which moisture substances are lost at 40.82-184.ll °C and the percentage of loss was 4.369%, while the second step was at 184.11-582.06 °C and the percentage of loss was 43.656%. As for the measurement of decomposition of cobalt complex, it was found that it decomposed in three steps, the first step included the loss of four molecules of water and oxygen, while the second step included the loss of 6H, nitrogen, and sulfur at 383.31-487.57 °C and the percentage of loss was 10.81% and the third step was at 487.57-572.17 °C and a percentage of loss was 31.127% [20] While Ni complexes for the other two decomposition steps refer to the loss of some complexes moiety in the range of 35.33-561.56 °C and the ratio of loss was 7.012%-41.794%.
Conclusion
The condensation reaction was done between 9,10- phenanthrenequinone and 2-mercaptoaniline in a 1:1 molar ratio produced a Schiff base ligand. Through its imine, carbonyl and thione groups, this ligand coordinates with Co(II) and Ni(II). This coordination took place at a ligand metal molar ratio of 1:1. The produced compounds were characterized by FTIR, UV-visible, thermal analysis, and molar conductance, magnetic susceptibility tests, and atomic absorption. The octahedral structure of the produced complexes is suggested by this data.
Acknowledgements
The authors would like to thank the Department of Chemistry, College of Science for Women, University of Babylon for providing all facilities to accomplish this work.
Conflict of Interest
The authors declare no conflict of interest.
Orcid:
Riyam Baqer Ibrahim: https://orcid.org/0009-0003-9375-2972
Suad T. Saad: https://orcid.org/0000-0002-7268-7345
----------------------------------------------------------------------
How to cite this article: Riyam Baqer Ibrahim*, Suad T. Saad. Cobalt (II) and Nickel (II) complexes with Schiff base derived from 9,10- phenanthrenquinone and 2-mercaptoaniline, synthesis and characterization, 2023, 5(8), 739-747. Link: https://www.echemcom.com/article_171377.html
----------------------------------------------------------------------
Copyright © 2023 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
