Document Type : Original Research Article
Authors
Department of Chemistry, College of Science, University of Al-Muthanna, Al-Samawah, Iraq
Abstract
This study of evaluation of Graphite Furnace Atomic Absorption Spectrometry GFAAS–Deuterium Background correction for lead determination, and application of this technique to assess whether the level of Pb(II) in whole blood is involved in pediatric, urinary tract patients, in the age range of 2-12 years old. Therefore, the research aimed to determine Pb(II) concentration in blood matrix children exposed to Pb(II) in their environmentally related daily activities by direct aqueous standardization. Blood samples for 18 patients were collected from Al-Samawah, maternity and children, Teaching Hospital, Al-Muthanna Province. 0.1 mL of blood samples were diluted by adding 0.9 mL matrix modifier (0.5% Vol. Triton X-100, 0.1% Vol. HNO3, and 0.2%w/v NH4H2PO4) and mixed properly, the same principle was applied for aqueous standard. L’vov platform was modified with 10 ppm Pd(NO3)2. For validation of results, spiking experiment analysis of four levels of samples has been used with good recovery results (102-104%). The gradients of the calibration curve, which were used below the CDC limit of BLLs, indicated that good linearity r = 0.9988 and the dynamic range was 10-30 μg.L-1. The BLLs were 11.5-18.4 µg.dL-1 for 18 samples of blood Pb(II) and LOD =0.28 µg/dL. The precision, as RSD, ranged between 2.46 and 8.56%. Accuracy and precision results imply they were sufficient to quantify blood Pb levels in the 10-30 μg/dL range. The evidence from recoveries analysis and children blood the samples were in excellent agreement with those obtained by ICP-MS and several GFAAS methods.
Graphical Abstract
Keywords
- Aqueous standardization
- children lead correlation with urea
- electrothermal atomizer
- lead
- urinary tract children patients
Main Subjects
Introduction
Lead is a non-essential element in the human body and was identified as toxic even at ultra-trace levels. However, in children and adults, its toxicity and biological role remain not widely understood [1,2].
A striking feature of Pb(II) is able to bind to many substrates and several SH- groups of proteins, DNA, blood albumin, and membrane of mitochondrial, resulting in generalized poisoning, a decrease of albumin levels, and inactivation of a wide range of enzymes [3].
In addition, lead has a high affinity for the Se element, which is playing as an adversary for this element [4]. Furthermore, Pb is competes with calcium ions in the biological fluid, which deactivate neurotransmitter release and decrease the density of bone minerals [5].
In general, Pb(II) cannot be metabolized in human cells. Hence, Pb(II) is gradually accumulated in organisms of human [6]. This can be an outcome in different health affects at definite exposure concentrations of lead [2, 10].
Consequently, the Pb(II) analysis in whole blood of children is attracting widespread interest due to its extremely toxic, mutagenic agents, and carcinogenic [11,12]. The Pb concentration in blood considered to be harmful of childhood is 25 µg/dL. However, in the United States, the elevated BLLs recently defined as at or above 5 µg/dL [13-15].
The Centre for CDC (Disease Control and Prevention) was adopted 10 μg/dL of pb as an advisory level [16]. In 2021, the CDC decreased the reference value of blood Pb yet further, from 10 μg.dL-1 to 3.5 μg.dL-1 [17].
Thus, only effective, selective, and sensitive analytical techniques could be utilized for blood lead determination under these criteria, for instance, the following spectroscopic techniques: atomic absorption spectroscopy (AAS) for all different modifications, flams, and flameless, primarily used the electrothermal atomization tandem AAS, because of its greater sensitivity and detection limit for Pb [18], inductive coupled plasma-mass spectroscopy ICP-MS [19-22], and x-ray fluorescence XRF [23].
Likewise, the electroanalytical techniques used for the Pb assessment in blood, such as striping voltammetry (SVA) and the analysis of neutron activation (NAA) but are rarely used for this purpose [24,25].
Regarding our knowledge, graphite furnace-AAS for sensitive and direct detection of Pb in children's blood specimens and urinary tract patients, has not been investigated. Hence, the aims of this work were: (i) To the investigate the amounts of Pb in children whole blood aged range of 2-12 years old at Al-Muthanna Province, Samawah City, Iraq and (ii) to set up preliminary results for Pb levels and compare to Creatinine and urea levels for pediatric Urinary tract patients.
Material and methods
Reagents
The reagents used and supplied had been analytical grade and from a high-quality company. All standards and reagents involved are listed below: TritonX-100, company of Fisher scientific, USA, has been utilized for preparation of rinse and as matrix modifier solutions. Ammonium Dihydrogen Phosphate (NH4H2PO4), Sigma Aldrich, Germany, has been utilized to matrix modifier and as co-injected modifier for background correction of deuterium. Palladium Nitrate and magnesium nitrate, Sigma Aldrich, are used as a permanent chemical modifiers. Nitric Acid (HNO3), Merck, Germany, has been utilized to prepare analytical curve, blank solution, and matrix modifier. 1000 µg.mL-1 Pb master standard, in solution reference material, was obtained from (Fluka, Switzerland) has been utilized to prepare working standards and for recovery of experiments. Phenol (Fluka, Switzerland) and Methanol (Sigma Aldrich, Germany) have removed any material around/on the autosampler tip. Finally, de-ionized (about 18 MΩ/cm) ultra-pure water has been used throughout.
Solution of matrix modifier: A 0.2% (w/v) NH4H2PO4 solution has been prepared by dissolving it in 0.1% (v/v) HNO3, and then mixing it with nonionic detergent, 0.5% (w/v) Triton X-100 in LDPE bottle.
Rinse solution: A washing solution, 104 % Triton X-100 has been prepared in water and filled it in an auto-sampler rinse bottle.
Standard preparation of Pb
Prepared the stock AAS of 1 mg/L Lead standard solution in 1% v/v concentrated HNO3
A 30 µg/L Lead, sub-standard, has been prepared in de-ionized water for calibration, the 30 µg/L was utilized to prepare a series of concentrations to consist of 1-3 µg/dL in 25 mL volumetric flasks. The 1 mg/L secondary standard has been the utilized to prepare a 10 µg/dL for spiking experiments of blood sample by diluting with matrix modifier solution.
Specimens Blood
A total 18, blood samples were collected for children of different ages from urology consultants in Al- Samawah, maternity and children, Teaching Hospital, Iraq, by professional nurses, Department of Laboratory, using virgin tubes (Vacutainer), containing potassium EDTA as anti-coagulant, free Pb. After gently rolling to enhance contact with the solution of EDTA, the samples were stored under refrigeration until assay. A completed blood sample for their patients has been centrifuged, for 15 min, to isolate serum from the residue of specimen, to assay the creatinine and urea. Microsoft Excel 2010 was used for statistical analysis, the results were expressed as mean ± standard deviation (mean ± SD) with an LSD test. Pearson's correlation was applied to determine the relationship among the present study parameters.
Absorbance measurements
The Shimadzu GFA (Japan), Atomic Absorption Spectroscopy has been used during this work, a GFA-7000 flameless accessory has a high accuracy, sensitivity, and is a recognized technique to Pb analysis in whole blood, as listed in Table 1. Many necessary parameters have been adjusted: Spectrometer, furnace, sampling, and calibration.
The measured signal is the transient height with a range (10.00-3.00) μg.dL-1. High-resolution peak was obtained with background correction, D2. The working volume was 20 µL, the fixed volume has been used for standard. Finally, the setup of the calibration method with external standards as the linear least squares fit.
Principle and calibration curve
The method for directly determining Pb in whole blood using Graphite Furnace AAS was essentially the same as that used by Parsons, with minor adjustments [26]. Background correction of the deuterium lamp was used throughout. A mixed solution of matrix modifier containing 0.5% Vol. Triton X-100, 0.1% Vol. HNO3, and 0.2%w/v NH4H2PO4 were utilized throughout, 0.1 mL portions of blood were diluted by adding 0.9 mL matrix modifier and mixed properly. Diluted blood samples aliquot of 20 µL, the small volume of sample injected was quickly heated all into the atomizer to convert it into an atomic vapor. The criteria for this work has been approved by adding a known amount of Pb to blood and calculating the recovery.
The aqueous calibration has been generated automatically, and the auto-sampler was set up to pipetted out 0.1 mL from each 30 µg/L Pb sub-standard; 10-30 µg/L, into 1.2 milliliter plastic cups, diluted all by adding 0.9 mL solution of matrix modifier with gently mixed. The final volume was 1000 µL, alongside transfer into an electro-graphite cuvette and the blank calibration in the same manner.
Results and discussion
Method Development
Background correction is significant for the run of real samples by GFAAS. The D2 lamp, continuum source for a background correction has been used when the mixed modifier and furnace program optimized. Significantly, the optimization of analytical methods was applied for the current protocol using a platform-type tube, and this technique allows lead to be atomized only when the inside of tube has reached atomization temperature. Since the D2 is a continuum source, the absorbance of its light by the blood's narrow absorption line is negligible.
In addition, the 0.5% Vol. Triton X-100, 0.1% Vol. HNO3, and 0.2%w/v NH4H2PO4 stabilize Pb atoms during pyrolysis (at more than 650 °C), and also breaks-up and increase the volatility of the interfering components of sample nonanalyte.
Background signal, with Pb signal from sample, resulted in fully shaped Pb peaks, remarkably with the same absorbance. An excellent way to avoid any possible interference of PO4-2 at the main line, an alternative line of Pb resonance of 283.3 nm was once used of lead resonance of 217 nm. Theoretically, 0.5% Triton X-100 + 0.2% w/v NH4H2PO4 + 0.1% HNO3 mixed modifier fluid dose not has any negative effects. Therefore, this modifier has been selected particularly for the Pb(II) analysis in blood samples with a D2 lamp furnace.
Method validation
Lead is a volatile substance, so the L'vov platform is used with integrated peak areas for absorbance measurements with matrix modifiers reagents provided in Table 2.
The type and amount of the reagent were studied to modify the atomizer surface, the results of this study have been decreased the pb volatility and enhanced the matrix volatility, as displayed in Figure 1.
An appropriate matrix modifiers solution has been introduced into electrothermal atomizer. Next, 1500-2500 °C was employed to pyrolysis.
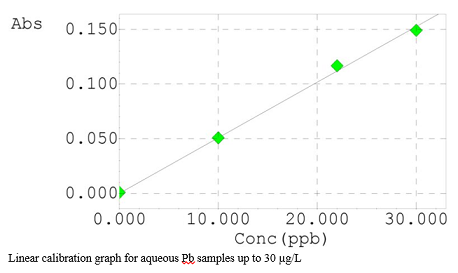
Aqueous calibration standards are suitable for Pb analyses in blood. As mentioned earlier, the experimental part, Table 1, the suitable temperatures were found for ash/atomize of the Pb with mixed modifiers. Pb volatilization in the blood matrix is delayed relative to the lead in the standard. This analysis highlighted that the combined solution of matrix modifier steady the Pb to 900 °C, which was applied in blood matrix, has been successful and resulted in a clean. The calibration graph of Pb is presented in Figure 3, which was constructed in an aqueous matrix, showing an R2 value ≥ 0.998, demonstrating the analysis linearity, exhibiting excellent linearity, up to 30 μg/L. In contrast, the peaks for all calibration standards and some samples are demonstrated in Figures 4 and 5. The dynamic range of aqueous standards 10-30 μg/L, the %R for all standards ranged from 97.7 to 104.3%, this elucidates the superb stability of the spectrometer parameters and background correction method.
These peak profiles revealed no significant difference between the peak shape of the aqueous standards and samples. This highlights no effects for the matrix sample in this analytical method. The detection limit of multiple measurements, 3SD, of the blood 1+9 diluent solution produced an estimated 0.28 µg/dL.
The spike work results show that the absorbance values gradient is the same between the blood and aqueous matrixes.
Because the analysis did not show any significant differences between the blood and standard aqueous samples, the direct analysis method of an aqueous standard can be used successfully. Thus, this situation indicated insignificant interference. The initial set of analyses indicated the impact of lead levels in children's blood specimens, Urinary tract patients, compared with creatinine and urea levels. The results for eighteen whole blood samples are provided in Table 3.
In addition, three replicate measurements of all blood samples, including different levels of Pb(II), a consistent result have been obtained throughout the experiment work, as presented in Table 4. Especially after atomization, no trace of any carbonaceous material was monitored inside the tube. The utilization of a fast ash ramp is thought to minimize carbon build-up by releasing vapor which is eliminated by the internal gas flow.
The recovery study to four blood samples is performed by spiking 10 μg/dL of the Pb, reference standard, to samples. The results of the recovery work for all samples are listed in Table 5, confirming that the proposed method can be used successfully, with full recovery of the spike.
Correlation studies
The second set of analyses revealed a significant positive correlation (R=0.79) of serum lead levels with the progress of age of children, urinary tract patients, as illustrated in Figure 6.
The study indicates that this group of children are markedly exposed to an additional amount of lead from different sources of many environmental pollutants with their age progress, In addition to the clear possibility of lead accumulation in the children's bodies.
Long-term lead exposure may cause a reduction in kidney function, which is an essential factor in assessing the detrimental health effects of lead. Different studies indicated that blood urea nitrogen and the correlation between creatinine levels and age was much stronger in groups that had been exposed to lead than in groups that had not [27].
The preliminary outcome of this study was to find out if there was a link between blood levels and each serum urea and creatinine levels in children, urinary tract patients. The study showed a moderate positively correlation of lead with urea levels (R=0.42) (Figure 7).
An earlier study hypothesized that repeated exposure to lead could result in the deposition of lead-protein complexes on the proximal tubules and glomerulus, which would impair urea and creatinine glomerular filtration and cause keep them in the plasma [28]. The results indicated that serum lead levels have a smooth positive correlation with creatinine levels in children, urinary tract patients (R= 0.17) (Figure 8).
Blood urea and creatinine levels were found to be abnormally high in correlation with blood lead levels during renal function tests. Furthermore, it appeared that blood urea was the most useful prognostic indicator for lead-induced renal impairment.Therefore, it should be a top priority to regularly monitor the kidneys of children exposed to lead, and this should be widely publicized.
The proposed work describes a GF-AAS technique for investigating whole blood lead levels in children using direct aqueous calibration.
Table 6 illustrates the present work and several analytical procedures for the whole blood lead levels analysis published over the last two decades, with Graphite Furnace Atomic Absorption Spectrometry being the preferred technique.
It illustrates the analytical figure of merit and whether with or without preliminary sample preparation.
Conclusion
Our work has led us to conclude that the system of D2-background correction can be utilized the determine lead accurately in high matrix samples, such as blood. However, the furnace parameters should be carefully controlled to include reading the maximum signal of the pb peak. The combination of the platform-type tube, co-injected 10 mg/L of Pd(NO3)2 with a Chemical modifier, 0.5% Vol. Triton X-100, 0.1% Vol. HNO3, and 0.2%w/v NH4H2PO4, provide an effective method for analyzing pb, a volatile element. This design of graphite tube allows blood sample atomization to take up to 900 heating cycles (RSD= 2.46-8.56%). In contrast, the chemical modifier reduced loss of the pb, the excellent recoveries obtained intimates that the sample introduction protocol followed was suitable and the matrix is not interfering with the final results. In addition, findings of this study indicate a positive correlation between the ages of patients with pb levels, moderate positively and smooth positive correlation with urea and creatinine levels of lead, respectively. Finally, despite the limitations, sampling was limited by the number of samples in the one age for a patient. Nevertheless, we believe our work could be a starting point for an enlarged of sampling range.
Acknowledgements
The financial support for this research was provided by Ministry of Higher Education Malaysia under FRGS Grant no. 2019-0147-103-02.
Conflict of Interest
The authors declare that there is no conflict of interest.
Orcid:
Zaman Sahb Mehdi: https://orcid.org/0000-0001-6114-9149
Noor Hameed Imran: https://orcid.org/0000-0001-7561-5307
--------------------------------------------------------------------------
How to cite this article: Zaman Sahb Mehdi*, Noor Hameed Imran, Talib A.H. Mousa, Hawraa Abdulkadhim Mazyed. Investigation of whole blood lead level in urinary tract children patients by effective method based on aqueous standardization using flameless atomic absorption spectrometry. Eurasian Chemical Communications, 2023, 5(9), 852-865. Link: https://www.echemcom.com/article_172140.html
--------------------------------------------------------------------------
Copyright © 2023 by SPC (Sami Publishing Company) + is an open access article distributed under the Creative Commons Attribution License(CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
