[1] S. Snapp, M. Rahmanian, C. Batello, T. Calls, Légumes secs et exploitations durables en Afrique subsaharienne,
FAO.,
2018, 68. [
Google Scholar], [
Publisher]
[2] R.A. Dixon, L.W. Sumner, Legume natural products: understanding and manipulating complex pathways for human and animal health,
Plant Physiol.,
2013,
131, 878-885. [
crossref], [
Google Scholar], [
Publisher]
[3] X. Peng, Z. Zheng, K.W. Cheng, F. Shan, G.X. Ren, F. Chen, M. Wang, Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts,
Food Chem.,
2008,
106, 475-481. [
crossref], [
Google Scholar], [
Publisher]
[4] J.D.H. Keatinge, W.J. Easdown, R.Y. Yang, M.L. Chadha, S. Shanmugasundaram, Overcoming chronic malnutrition in a future warming world: the key importance of mungbean and vegetable soybean,
Euphytica.,
2011,
180, 129-141. [
crossref], [
Google Scholar], [
Publisher]
[5] J.E. Arsenault, R.J. Hijmans, K.H. Brown, Improving nutrition security through agriculture: an analytical framework based on national food balance sheets to estimate nutritional adequacy of food supplies,
Food Secur.,
2015,
7, 693-707. [
crossref], [
Google Scholar], [
Publisher]
[6] C.H. Foyer, H.M. Lam, H.T. Nguyen, K.H. Siddique, R.K. Varshney, T.D. Colmer, W. Cowling, H. Bramley, T.A. Mori, J.M. Hodgson, J.W. Cooper,
Neglecting legumes has compromised human health and sustainable food production,
Nat. Plants,
2016,
2, 16112. [
crossref], [
Google Scholar], [
Publisher]
[7] M. Khalil, S. Noor, Z. Ahmad, F. Ahmad, Fate of Pakistani exported Mango due to its toxicity (heavy metals, pesticides, and other toxic organic components),
J. Appl. Organomet. Chem.,
2023,
3, 86-107. [
crossref], [
Google Scholar], [
Publisher]
[8] Y. Yao, X. Yang, J. Tian, C. Liu, X. Cheng, G. Ren, Antioxidant and antidiabetic activities of black mung bean (Vigna radiata L.),
2013,
J. Agric. Food Chem.,
61, 8104–8109. [
crossref], [
Google Scholar], [
Publisher]
[9] J. Luo, W. Cai, T.B. Wua, B. Xu, Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiata L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities,
Food Chem.,
2016,
201, 350-360. [
crossref], [
Google Scholar], [
Publisher]
[10] J.P. Singh, A. Kaur, N. Singh, L. Nim, K. Shevkani, H. Kaur, D.S. Arora, In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols,
LWT-Food Sci. Technol.,
2016,
65, 1025-1030. [
crossref], [
Google Scholar], [
Publisher]
[11] H.H. Hama, I. Moussa, K. Ikhiri, B. Ouedraogo, R. Adama, Activité antioxydante des extraits méthanoliques de differents organes de Detarium microcarpum,
European Sci. J.,
2019,
15, 1857-7881. [
crossref], [
Google Scholar], [
Publisher]
[12] G. Lal Gupta, N. Patil Samant, Acute and sub-acute oral toxicity evaluation of avicularin,
J. Med. Chem. Sci.,
2023,
6, 2327-2337. [
crossref], [
Pdf], [
Publisher]
[13] L.S. Lai, S.T. Chou, W.W. Chao, Etudes sur l’antioxactivités idatives de Hsian-tsao (Mesona procumbens Hemsl) feuille de gomme,
J. Agri. Food Chem.,
2001,
49, 963-968. [
crossref], [
Google Scholar], [
Publisher]
[14] N.A. Patil, S. Udgire, D.R. Shinde, P.D. Patil, Green synthesis of gold nanoparticles using extract of Vitis vinifera, Buchananialanzan, Juglandaceae, Phoenix Dactylifera plants, and evaluation of antimicrobial activity,
Chem. Methodol.,
2023,
7, 15-27. [
crossref], [
Pdf], [
Publisher]
[15] R.E. Wrolstad, T.E. Acree, E.A. Decker, M.H. Penner, D.S. Reid, S.J. Schwartz, C.F. Shoemaker, D. Smith, P. Sporns, Handbook of food analytical chemistry pigments, colorants, flavors, texture, and bioactive food components,
John Wiley and Sons, Inc.,
2005, 81-90. [
Google Scholar], [
Publisher]
[16] A.H. Moumouni Koala, K. Somé, E. Palé, A. Sérémé, J. Belem, M. Nacro, Evaluation of Eight Orange Fleshed Sweetpotato (OFSP) Varieties for Their Total Antioxidant, Total Carotenoid and Polyphenolic Contents
, J. Nat. Sci. Res.,
2013,
3, 67-72. [
Google Scholar], [
Publisher]
[17] O. Mahamadi, H. Adama, W.F.M. Serge, J.T. Benoit, P. Eloi, N. Mouhoussine, Détermination des paramètres physico-chimiques et des teneurs en micronutriments antioxydants de dix variétés de mung bean (Vigna radiata) produits dans les conditions agroécologiques du Burkina Faso,
J. Chem. Biol. Phy. Sci.,
2021,
11, 254-274. [
crossref], [Pdf], [
Publisher]
[18] G. Chala, Review on green synthesis of iron-based nanoparticles for environmental applications,
J. Chem. Rev.,
2023,
5, 1-14. [
crossref], [
Pdf], [
Publisher]
[19] A.R. Maleki, L. Nateghi, P. Rajai, The Effect of microwave pretreatment on the extraction rate of flavonoid, anthocyanins, antioxidant compounds and antimicrobial activity of Punica Granatum Var. Pleniflora (Persian Golnar),
Chem. Methodol.,
2022,
6, 280-292. [
crossref], [
Google Scholar], [
Publisher]
[20] Y. Jun, F. Lingling, X. Jian, X. Yedan, Ultrasound-assisted extraction of corn carotenoids in ethanol,
Int. J. Food Sci. Technol.
, 2011,
46, 2131-2131. [
crossref], [
Google Scholar], [
Publisher]
[21] J. Mc Murry, Organic Chemistry, 7th Edition, Thomson Brooks/ Cole., 2008, 504 chapter 14.
[22] P. Sáez-Plaza, M.J. Navas, S. Wybraniec, T. Michałowski, A.G. Asuero, An overview of the Kjeldahl method of nitrogen determination. part II. sample preparation, working scale, instrumental finish, and quality control,
Crit. Rev. Anal. Chem.,
2013,
43, 224-272. [
crossref], [
Google Scholar], [
Publisher]
[23] B. Ou, R.L. Prior, D. Huang, The chemistry behind dietary antioxidant capacity assays,
J. Agri. Food Chem.
, 2005,
53, 1841-1856. [
crossref], [
Google Scholar], [
Publisher]
[24] A. Hatami, Preparation, Description and evaluation of the lethality acid loaded liposomal nanoparticles against in vitro colon and liver cancer,
J. Chem. Rev.,
2021,
3, 121-133. [
crossref], [
Google Scholar], [
Publisher]
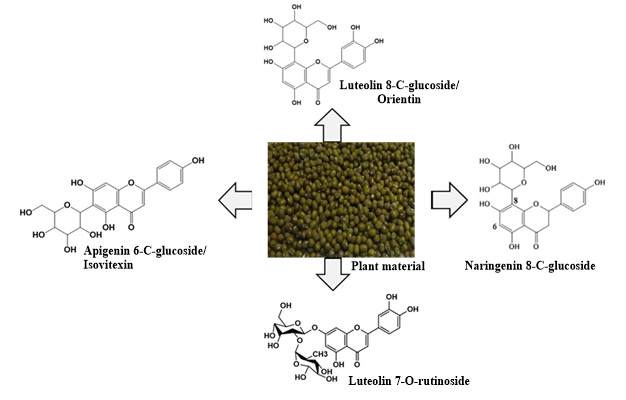
[25] M. Ouedraogo, A. Hema, B.S.R. Bazié, S.W. Zida, B.J.T. Batieno, E. Kabré, E. Palé, M. Nacro, Identification of major flavonoids using HPLC-MS/MS and determination of antioxidant potential of Vigna radiata seed extracts,
Journal de la Société Ouest-Africaine de Chimie.,
2021,
050 57-67. [
Google Scholar], [
Publisher]
[26] A. Mbaiogaou, A. Hema, M. Ouedraogo, E. Palé, M. Naitormbaide, Y. Mahamout, M. Nacro, Etude comparative des teneurs en polyphénols et en antioxydants totaux d’extraits de graines de 44 variétés de voandzou (Vigna subterranea (L.)Verdcourt),
Int. J. Biol. Chem. Sci.
, 2013,
7, 861-871. [
crossref], [
Google Scholar], [
Publisher]
[27] N. Parisa, M.T. Kamaluddin, M.I. Saleh, E.S. Sinaga, R. Umi Partan, I. Irfannuddin, Flavonoids as Antioxidants: A Review on Tempuyung Plant (Sonchus Arvensis),
J. Med. Chem. Sci.,
2023,
6, 2310-2318. [
crossref], [
Pdf], [
Publisher]
[28] Z. Mohammedi, F. Atik, Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) karst,
Int. J. Pharma. Bio. Sci.,
2011,
2, 609-615. [
Google Scholar], [
Publisher]
[29] N. Trabelsi, W. Megdiche, R. Ksouri, H. Falleh, S. Oueslati, B. Soumaya, H. Hajlaoui, C. Abdelly, Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves,
LWT-Food Sci. Technol.,
2010,
43, 632-639. [
crossref], [
Google Scholar], [
Publisher]
[30] F.I. Ahmadi, R. Fathollahi, D. Dastan, Phytochemical constituents and biological properties of Scutellaria Condensata Subsp, Pycnotricha,
J. Appl. Organomet. Chem.,
2022,
2, 119-128. [
crossref], [
Google Scholar], [
Publisher]
[31] F.J. He, G.A. Macgregor, Beneficial effects of potassium on human health,
Physiol Plant.,
2008,
133, 725-735. [
crossref], [
Google Scholar], [
Publisher]
[32] V.E. Kemi, M. Kärkkäinen, C.J. Lamberg-Allardt, High phosphorus intakes acutely and negatively affect Ca and bone metabolism in a dose-dependent manner in healthy young females,
British J. Nut.,
2006,
96, 545-552. [
crossref], [
Google Scholar], [
Publisher]
[33] J. Sochor, M. Ryvolova, O. Krystofova, P. Salas, J. Hubalek, V. Adam, L. Trnkova, L. Havel, M. Beklova, J. Zehnalek, I. Provaznik, Fully automated spectrometric protocols for determination of antioxidant activity: advantages and disadvantages,
Molecules,
2010,
15, 8618-8640. [
crossref], [
Google Scholar], [
Publisher]
[34] D. Cao, H. Li, J. Yi, J. Zhang, H. Che, J. Cao, L. Yang, C. Zhu, W. Jiang, Antioxidant properties of the mung bean flavonoids on alleviating heat stress,
PLoS. One,
2011,
6, 2010-2071. [
crossref], [
Google Scholar], [
Publisher]
[35] L.L. Saldanha, W. Vilegas, A.L. Dokkedal, Caractérisation des flavonoïdes et des acides phénoliques dans Myrcia Bella cambess. Utilisation de FIA-ESI-IT-MSn et HPLC-PAD-ESI-IT-MS combinés à la RMN,
Molécules.,
2013,
18, 8402-8416. [
crossref], [
Google Scholar], [
Publisher]
[36] M.F. Lubis, V.E. Kaban, K. Gurning, P. Parhan, H. Syahputra, N.A. Juwita, R. Astyka, I. Zulfansyah, Phytochemicals and biological activities of ethanolic extract of Garcinia atroviridis leaf grown in indonesia,
J. Med. Chem. Sci.,
2023,
6, 2456-2469. [
crossref], [
Pdf], [
Publisher]
[37] F. Laëtitia, Signature moléculaire de milieux complexes : Stratégie de couplage à la spectrométrie de masse et interprétation des données, Thèse de Doctorat. Université d’Orléans,
2019, 504-674. [
Google Scholar], [
Publisher]
[38] W. Patrice, W. Jean-Luc, N. Karine, R.H. Kirsten, J.M. Hilary, H. Kurt, Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of Cglycosidic flavonoid isomers,
J. Chromatogr. A.,
2001,
926, 29-41. [
crossref], [
Google Scholar], [
Publisher]
[39] V. Vukics, V. Guttman, Caractérisation structurale des glycosides flavonoïdes par spectromètre de masse à plusieurs étages y,
Mass Spectrometry Reviews,
2010,
29, 1-16. [
crossref], [
Google Scholar], [
Publisher]
[40] F. Cuyckens, Y.L. Ma, G. Pocsfalvi, M. Claeysi, Stratégies spectrales de masse en tandem pour la caractérisation structurale des glycosides flavonoïdes,
Analusis.,
2000,
28, 888-895. [
crossref], [
Google Scholar], [
Publisher]
[41] W.O. Jean Claude, K. Moumouni, O. Noufou, B.K. Félix, G. Pascal, L.B.C. Yvonne, Total phenolics and total flavonoid contents, antioxidant activity and flavonoids identification by high-performance liquid chromatography-tandem mass spectrometry of odontonema strictum (Acanthaceae) leaves, Asian J. Plant Sci. Res., 2017, 7, 54-63.
[42] E. Chan, S. Yap, A. Lau, P. Leow, H. Koh, Ultra-performance liquid chromatography/ time-of-flight mass spectrometry based metabolomics of raw and steamed Panax notoginseng,
Rapid Commun. Mass Spectrom.,
2007,
21, 519-528. [
crossref], [
Google Scholar], [
Publisher]
[43] B. Abad-García, S. Garmón-Lobato, L.A. Berrueta, B. Gallo, F. Vicente, New features on the fragmentation and differentiation of C-glycosidic flavone isomers by positive electrospray ionization and triple quadrupole mass spectrometry,
Rapid Commun. Mass Spectrom.,
2008,
22, 1834-1842. [
crossref], [
Google Scholar], [
Publisher]