Document Type : Original Research Article
Authors
1 Guilan university
2 Urmia university
Abstract
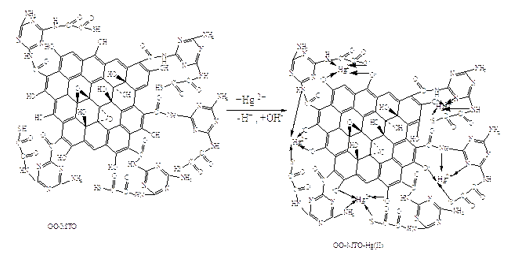
In this study a new method by Application of graphene oxide Nano sheets– Melamine –TioOxalic acid composite was exhibited as adsorbent for the elimination of toxic mercury (II) ions from aqueous solutions. The combine has the authority to adsorb the organic and inorganic combines. Through the immobilization of Melamine-TioOxalic acid (MTO) onto graphene oxide nanosheets, the desired composite was synthesized and introduced by field emission scanning electron microscopy (FE-SEM), UV-Vis and Fourier transform infrared (FT-IR) spectroscopic techniques. The different experimental parameters such as pH and concentration of the aqueous solution of Mercury (II), amounts of the MTO and the graphene oxide (GO), temperature, contact time, have been optimized. It was presented that they carry out the efficiency of Mercury (II) ion importantly, increased after immobilization of MTO on the graphene oxide Nano sheets. GO-MTO is a very suitable adsorbent for removing of heavy metal ions especially Mercury (II) ions. Mercury and Gold have very interesting for binding with sulfur.
Graphical Abstract
Keywords